On January 28, 2026 (local time), a research team led by Xu Huaqiang, researcher at the Center for Translational Medicine and Structural Biology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, together with colleagues from the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, published a research article entitled “Structures of Ostα–β reveal a unique fold and bile acid transport mechanism” in Nature. By deeply integrating cryo-electron microscopy (cryo-EM) structural determination, molecular dynamics simulations, and electrophysiological functional analyses, the study systematically elucidates the unique three-dimensional architecture, substrate recognition mode, and transmembrane transport mechanism of Ostα/β. These findings provide critical evidence that resolves a long-standing mechanistic question in the field.
Bile acids are vitally important to the human body and can be seen as true “multitaskers.” They play indispensable roles in digestion and nutrient absorption, energy metabolism, and the maintenance of hormonal signaling homeostasis. Bile acids continuously circulate between the liver and the intestine, forming the finely tuned enterohepatic circulation, which relies on the coordinated action of a series of membrane transporters. The smooth operation of this cycle depends on these “transport helpers,” each performing distinct but complementary roles to enable the bidirectional movement of bile acids. Over the past decades, most of the transporters involved have been identified and their mechanisms relatively well characterized. However, one critical question has remained unresolved: how are bile acids efficiently exported from the basolateral membrane of intestinal epithelial cells (enterocytes) into the portal circulation to continue the cycle?
To understand this, we can first look at the classic paradigm of bile acid handling in hepatocytes. At the sinusoidal membrane (the “front door” of hepatocytes), sodium-dependent or facilitated transporters mediate bile acid uptake, while at the canalicular membrane (the “back door”), ATP-binding cassette (ABC) transporters actively export bile acids using energy. It was once hypothesized that a similar “input–output” logic operates in other epithelial tissues such as the intestine. However, the discovery in 2004 of the organic solute transporter Ostα/β challenged this assumption. Rather than being a single transporter, Ostα/β is a heterodimer composed of Ostα and Ostβ subunits. Subsequent studies established it as the key effector responsible for bile acid efflux across the basolateral membrane of enterocytes (as shown in Fig. 1A, B). Yet how exactly it works has remained unclear.
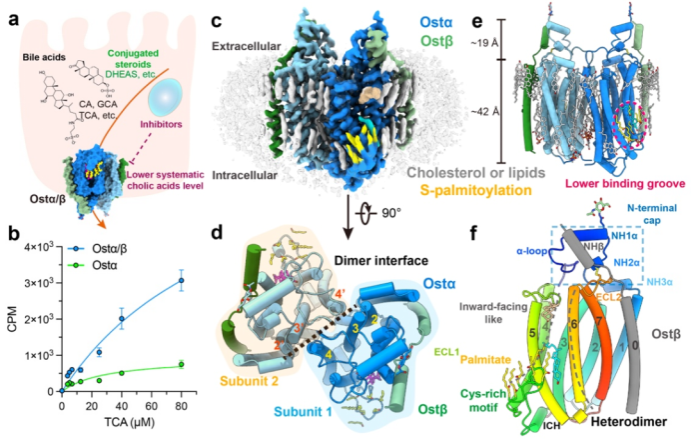
Figure 1. Assembly and overall structure of the human Ostα/β tetramer
The present study systematically reveals the structural basis and functional mode of Ostα/β from both structural and mechanistic perspectives. At atomic resolution, it fills in a long-missing and critical link in the enterohepatic circulation—bile acid efflux—providing definitive answers to a problem that has perplexed the field for years, and offering important insights for the precise intervention of related diseases.
Disorders of bile acid metabolism constitute an important pathological basis for many hepatobiliary diseases, including cholestasis and non-alcoholic fatty liver disease. The in-depth elucidation of the structure and transport mechanism of Ostα/β makes it, for the first time, a potential therapeutic target with a clearly defined structural framework and regulatory mode. From a translational perspective, targeted modulation of Ostα/β transport activity or direction could allow fine-tuned control of bile acid distribution between the liver and intestine under different pathological conditions. This strategy may help alleviate cholestasis, reduce bile acid–mediated hepatotoxicity, and improve associated metabolic abnormalities. It thus represents a shift in therapeutic thinking—from indirect modulation of metabolic pathways to more precise, direct intervention at a key transport step—offering an entirely new treatment paradigm for bile acid–related diseases.
In addition, structural comparison analyses revealed that Ostα/β shares significant topological similarity with the TMEM184 protein family, whose functions are not yet fully defined. This suggests that TMEM184 family members may belong to a new class of transporters rather than conventional membrane receptors, opening up new directions for re-evaluating their biological roles and potential disease associations.
In this study, the team successfully expressed and purified the human Ostα/β complex in mammalian cells and determined its structure at a resolution of 2.6–3.1 Å using single-particle cryo-EM (Fig. 1C). Structural analysis showed that Ostα/β does not exist as isolated units, but assembles into a symmetric tetramer composed of two Ostα–Ostβ heterodimers (Fig. 1D, E). The Ostα subunit adopts a previously unknown seven-transmembrane-helix fold (Fig. 1F) that shows no significant homology to any known transporter family. The Ostβ subunit contributes a single transmembrane helix that lies adjacent to the seventh helix of Ostα, stabilizing the core architecture “side by side.” This unique structural organization explains why Ostα/β is classified as a distinct transporter family, SLC51.
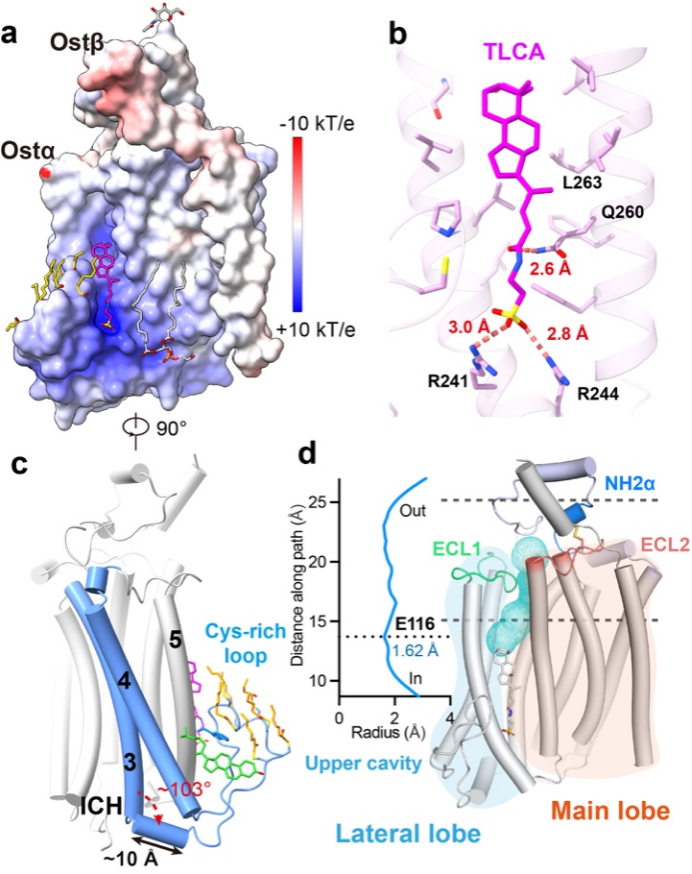
Figure 2. Lateral substrate-binding pocket and channel of Ostα/β
Further structural analyses revealed a laterally open substrate-binding groove within the membrane near the cytoplasmic side. This groove is formed by a cysteine-rich intracellular loop and is extensively palmitoylated (Fig. 2A, C). Together with hydrophobic amino acid residues, these lipid modifications create a local microenvironment well suited for binding amphipathic steroid molecules. The team resolved high-resolution structures of Ostα/β bound to two physiological substrates—taurolithocholic acid (TLCA) (Fig. 2B) and dehydroepiandrosterone sulfate (DHEAS). The structures clearly show that positively charged arginine residues (R241 and R244) within the groove form specific electrostatic interactions with the sulfonate groups of the substrates, conferring selectivity for negatively charged molecules. Beyond this static binding site, analysis of the three-dimensional density maps and structural features identified a hydrophilic channel extending from the basal binding groove toward the extracellular side (Fig. 2D). Combined with molecular dynamics simulations, the study proposes that substrates may traverse the membrane through this channel.
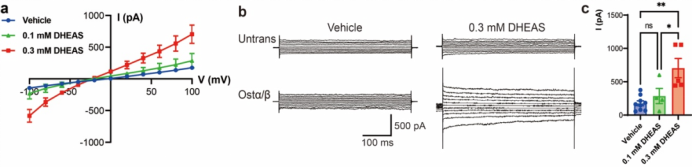
Figure 3. Voltage-sensitive transport by Ostα/β
Structural insights and hypotheses alone are not sufficient; the team therefore conducted dynamic experiments to validate the transport process. For the first time, exploiting the charged nature of bile acid derivatives, they used whole-cell patch-clamp electrophysiology to directly record membrane potential–dependent transmembrane currents triggered by substrate (DHEAS) application in cells expressing Ostα/β (Fig. 3). This approach converts bile acid transport into a measurable electrical signal, enabling real-time and controlled observation. The experiments confirmed that Ostα/β functions as a facilitated diffusion transporter whose transport direction is not fixed, but instead determined by the electrochemical gradients of the substrate across the membrane. Specifically, membrane potential acts as a key regulatory factor that biases the transport direction: depolarization favors substrate influx, whereas hyperpolarization promotes efflux. Thus, membrane potential is not merely a passive background parameter, but a decisive factor that biases bidirectional transport, enabling Ostα/β to preferentially mediate bile acid efflux under different physiological conditions.
Professor Ma Xiong from Renji Hospital is a co-corresponding author of the study. The co–first authors are Dr. Yang Xuemei (postdoctoral fellow, Center for Translational Medicine and Structural Biology, Ruijin Hospital), Dr. Cui Nana (postdoctoral fellow, Renji Hospital), Li Tianyu (assistant researcher, Shanghai Institute of Materia Medica, Chinese Academy of Sciences), and He Xinheng (former PhD graduate of the Shanghai Institute of Materia Medica).

