Shanghai Jiao Tong University School of Medicine remains closely aligned with national strategic priorities, committed to advancing the frontiers of global science and technology,addressing major national needs, and safeguarding public health. Guided by problem-oriented and demand-driven principles, and propelled by major scientific missions, the university continues to strengthen organized researchinitiatives, mobilize resourcesto pursueoriginal and pioneering scientific breakthroughs, and enhance the overall effectiveness of scientific innovation. Recently, multiple affiliated hospitals havereported significant research findingspublishedin leading international academic journals.
Professors Liu Jun and Kang Wenyan from Ruijin Hospital Publish New Advances in Alzheimer’s Disease Pharmacotherapy

A research team led by Professors Liu Jun and Kang Wenyan from the Department of Neurology at Ruijin Hospital recently published a study titled “Safety and Effectiveness of Lecanemab in Chinese Patients with Early Alzheimer’s Disease: Evidence from A Multidimensional Real-world Study.” This work represents the first real-world study of lecanemab conducted in China, made possible through the special regulatory policies of the Boao Lecheng International Medical Tourism Pilot Zone and the integrated operation of Ruijin Hospital and its Hainan campus.
Alzheimer’s disease (AD), adevastating neurodegenerative disorder, affects approximately 55 million people worldwide, including nearly 10 million patients in China.As the disease progresses, patients gradually lose cognitive and behavioral functions. Before the emergence of disease-modifying therapies (DMTs), ADtreatment was largelylimited tosymptomatic management, with no effectiveinterventions todelay progression. In September 2023, lecanemab, a targeted therapy for Alzheimer’s disease (AD), was launched in the Lecheng International Medical Tourism Pilot Zone. It specifically eliminates amyloid-beta (Aβ) proteins, the toxic proteins that drive the onset and progression of Alzheimer’s disease, delays disease progression, and is the world’s first causal therapy for the condition.
As the first real-worldstudy of lecanemabconductedin Chinese patients with early-stage AD under China’s“pilot and early access” policy framework, this study bridges the evidence gap between international clinical trial data and clinical practice in the Chinese population. It provides important insights into the safety and preliminaryefficacy of lecanemab in patients with mild cognitive impairment (MCI) and mild AD, offering valuableevidence to inform future clinical practice and long-term monitoring strategies. The findingsfurtherconfirm that lecanemab effectively slows cognitive decline and reduces cerebral Aβ deposition in Chinese patients with early-stage AD.
Renji Hospital and SJTU Computer Science Team Develop New AI Prognostic System for STEMI
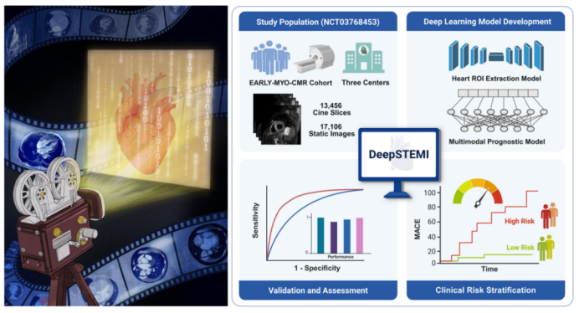
A collaborative team led by Professor Bu Jun from the Department of Cardiology at Renji Hospital and Professor Sheng Bin from the School of Computer Science at Shanghai Jiao Tong Universityhas recentlypublished an original research article titled “A Novel Deep Learning System for STEMI Prognostic Prediction from Multi-Sequence Cardiac Magnetic Resonance” onlineinScience Bulletin, a leading multidisciplinary journal (CAS Zone 1, TOP journal, IF = 21.1). Leveraging a prospective multicentercardiac imaging cohort (NCT03768453), the research team developed DeepSTEMI, a novel AI-based prognostic prediction system thatutilizes cardiac imaging to accurately assess the risk of future cardiovascular events in patients with acute myocardial infarction. By integrating andinterpretingmulti-source imaging features, the DeepSTEMI system enables fully automated and intelligent risk stratification,offering a new technological tool for the precision management of patients with acute myocardial infarction.
DeepSTEMI is the first fully automated multimodal deep learning system designed for prognostic risk assessment in STEMI patients. It integrates interpretable U-Net and Transformer architectures for multi-sequence cardiac MRI segmentation, jointly modeling multimodal MRI data with clinical variables. The system also introduces two innovative modules,the Hierarchical Feature Fusion Module (HFFM) and the Missing Modality Feature Generation Module (M2FGM), to enable efficient cross-modality fusion and robust prediction even when imaging modalities are incomplete. The study analyzed over 30,000 MRI images from real-world, multicenter datasets, providing strong evidence for the model’s generalizability. By enablingmore accurate long-term cardiovascular event risk prediction and intelligent risk stratification, DeepSTEMIhighlights the powerful potential of artificial intelligence in advancing precision cardiovascular medicine.
In a multicenter external validation study, DeepSTEMI demonstrated markedly superior predictive performance compared with existing clinical scoring systems and traditional imagingmetrics, enabling clear discrimination between high-riskand low-risk patients. Notably, in risk stratification, the system was able to identify patients likely to experience future adverse events earlier and with greater precision, far surpassing conventional models in its risk‑alerting capability. Thiscapability facilitates earlier detection andtimelyintervention for high‑risk STEMI patients, improves clinical outcomes, and underscores the system’s substantial clinical value. Moreover, DeepSTEMI maintained robust performance across multiple hospitals and various types of MRI scanners, demonstrating strong cross‑center and cross‑device generalizability and establishing a solid foundation for clinical implementation.
Researcher Zhao Jianyuan from Xinhua Hospital Joins Hands with Multiple Research Teams to Publish Landmark Study inCell Metabolism

Recently, a multidisciplinary research team led by Researcher Zhao Jianyuan from Xinhua Hospital, in collaboration with Professor Jin Liping from the Obstetrics and Gynecology Hospital of Fudan University and experts from four major tertiary hospitals in Shanghai, published a landmark study inCell Metabolism. The studyrevealed, for the first time, the molecular mechanism by which dysregulated TMAO metabolism in decidual tissuecontributes to recurrent spontaneous abortion (RSA), offering newprospects for precisiontherapeutic strategies in patients with unexplained RSA.
“A successful pregnancy depends on both a healthy embryo and a receptive uterine environment,” the research team explained. Decidualization of the endometrium is acritical physiological process thatprimes the uterus for embryo implantation, functioninglike a “comfortable cradle” for the developing embryo. Under hormonal and other signaling cues, endometrial stromal cells undergo a remarkable transformation, differentiating into mature decidual cells that secrete nutrients and immune‑tolerant factors. These changes provide essential support and protection for the “new arrival”, the embryo, ensuring a stable environment for early development. Abnormal decidualization, however, is one of the major causes of implantation failure and early pregnancy loss.
To address this question, the research team turned its attention to theconnection between metabolic dysregulation and decidualization. Metabolomic analysis of clinical sampleshighlighted a key molecule—trimethylamine N‑oxide (TMAO). By comparing tissue samples from women undergoing elective termination and those with recurrent spontaneous abortion (RSA), the researchers identified a striking difference: the TMAO metabolic pathway was markedly downregulated in the decidual tissue of RSA patients, while chorionic tissue and peripheral blood showed no notable abnormalities. This finding suggests that a uterus‑specific defect in TMAO metabolism may be a critical culprit underlying pregnancy loss.
To validate this hypothesis, the team conducted both in vitro cellular experiments and in vivo animal studies. The results were striking: when pregnant mice were fed a choline-deficient diet or their TMAO-synthesizing enzymes were inhibited, the embryosexhibited a markedly increased resorption rate and reduced fetal weight, manifestingpronounced adverse pregnancy phenotypes. In other words, the “cradle” was malfunctioning, preventing the “little guest”, the embryo, from developing safely. In contrast, exogenous TMAO supplementation not only effectively promoted decidualization of endometrial stromal cells but also successfully rescued the miscarriage phenotype, therebyrepairing key components of the impaired “cradle” and restoring a stable developmental environment for the embryo. These findings establish the essential role of TMAO in maintaining normal pregnancy.
More importantly, this fundamental discovery has demonstrated clear translational potential. When the research team isolated endometrial stromal cells from RSA patients and conducted in vitro experiments, they found that TMAO supplementation was able to rescue the decidualization process in 15% of patients. This suggests that for RSA patients whose condition is driven by insufficient TMAO synthesis, TMAO supplementation may offer a precise and straightforward therapeutic strategy.
Ninth People’s Hospital Team Publishes AI Breakthrough inDigital Medicine

A team led by Professor Tonetti from the Institute of Oral Craniofacial and Sensory Health and the Center for Periodontal and Implant Innovation at Shanghai Ninth People’s Hospital published a study in Digital Medicine, a Nature partner journal. The study systematically evaluated the deep learning model HC Net+ for diagnosing periodontitis using panoramic radiographs in real-world multicenter clinical settings. HC Net+ achieved a diagnostic accuracy of 94.2%, outperforming periodontal specialists and enabling primary-care dentists to reach specialist-level diagnostic performance.
Periodontitis, one of the most prevalent chronic oral diseases worldwide, is often referred to as the “silent killer of teeth.” Its early symptoms are subtle and easily overlooked, while advanced disease can lead to tooth mobility and eventual tooth loss, severely compromising oral health. As a major global public health concern, the effective early detection of this condition remains challenging. Currently, periodontal probing the standard clinical method, is invasive, time-consuming, and highly dependent on clinician experience. Meanwhile, panoramic radiographs, though widely used, make early bone loss difficult to detect with the naked eye, and variations in imaging equipment further affect diagnostic consistency. These limitations make it challenging to meet the needs of large-scale population screening.
To address these challenges, Professor Tonetti’s team, together with collaborating groups, iteratively developed the HC Net+ deep learning model, the first AI system designed specifically for clinical panoramic radiographs and validated across multiple centers and multiple diagnostic standards for periodontitis detection.
This study presents an efficient, accurate, and highly scalable new tool for screening periodontitis. According to the research team, the core strengths of HC Net+ lie in its high efficiency, precision, and ease of deployment. It requires no additional equipment investment for primary care institutions and can deliver high-quality periodontitis screening using existing panoramic radiographs alone, effectively lowering diagnostic barriers and reducing patient discomfort. This makes widespread adoption in community health centers and remote regions feasible. The team noted that future work will continue to advance AI-driven periodontal disease diagnostics, promoting screening that is more efficient, accessible, and precise.
Immune Age Diagnostic Kit Project by Professor Wang Honglin’s Team Selected for SJTU’s Fifth “Top Ten Scientific Advances”

Recently, the project “Translational Application of an Immune Age Diagnostic Kit” led by Professor Wang Honglin from Shanghai General People’s Hospital was selected as one of the Top Ten Scientific Advances of Shanghai Jiao Tong University in its fifth annual awards. The project, for the first time, identified a key immune cell subset serving as a marker of immune age, achieved a record-breaking technology transfer agreement, and successfully transitioned from original discovery to industrial translation, representing a milestone breakthrough in immune assessment and health management.
Traditional immune testing primarily relies on quantifying major immune cell subsets, such as CD3+ and CD4+, which often fail to accurately reflect the overall functional state orimmunological aging of the system. There is apressing need in clinical practice and health management toconvertthe abstract and complex concept of “immunity” into aquantifiable and easilyassessable objective indicator.
To address this challenge, Professor Wang’s team adopted an innovative approach by focusing on the more stable bone marrow immune system. Through high-throughput single-cell sequencing of bone marrow samples from healthy individuals aged 3 to 91, the team generated a detailed atlas of age-related immune cell changes and, for the first time globally, identified a novel T cell subset, TSN cells, that is highly associated with immune aging. The quantity and quality of TSN cells were shown to be specific biological markers directly indicating immune aging and functional status. This core indicator, initially identified in bone marrow, was subsequently validated in a large number of peripheral blood samples, thereby forming the scientific foundation for the diagnostic kit.
Over the three-and-a-half-year research period, Professor Wang’s teamclosely collaboratedwith the hospital’s translational medicine department and its strategic industry partner, GuangdongTaienkang Pharmaceutical Co., Ltd. This deepcollaboration ensured that the research direction consistently addressed real-world clinical needs. Owing to itsremarkable innovation and translational potential, the project’sassociated patents were licensed toTaienkang Pharmaceutical in October 2023 for a total contract value of 600 million RMB, serving as a model of transforming “research patents” into “patient benefits.”
Professor Wan Xinjian’s Team from Shanghai Sixth People’s Hospital Publishes New IBD Findings inAdvanced Science

Recently, a research team led by Professor Wan Xinjian from the Department of Gastroenterology at Shanghai Sixth People’s Hospital published a study titled “ETV1 Drives CD4⁺ T Cell Mediated Intestinal Inflammation in Inflammatory Bowel Disease Through Amino Acid Transporter Slc7a5” online in the internationally renowned journal Advanced Science (IF = 14.1). The study systematically demonstrates that the transcription factor ETV1 directly regulates the amino acid transporter SLC7A5, driving metabolic reprogramming and functional activation of CD4⁺ T cells, thereby promoting intestinal inflammation. These findings provide new mechanistic insights and potential therapeutic strategies for the precision treatment of inflammatory bowel disease (IBD).
IBD is a chronic, relapsing inflammatory disorder of the gastrointestinal tract with no curative treatment currently available. Abnormal activation and proliferation of CD4⁺ T cells, including subsets such as Th17 cells, are central to its pathogenesis. Activated T cells require a substantial uptake of amino acids and other nutrients to support rapid proliferation and immune effector functions; however, the precise regulatory mechanisms governing this metabolic process have remained unclear.
The study first revealed that ETV1 expression is significantly upregulated in the inflamed intestinal mucosa of IBD patients and positively correlates with disease severity. Further experiments demonstrated that targeted inhibition of ETV1 significantly alleviated TNBS-induced experimental colitis in mice, as evidenced by reduced weight loss, shorter colon length, decreased inflammatory cell infiltration, and lower levels of pro-inflammatory cytokines. Moreover, adoptive transfer experiments demonstrated that ETV1-deficient CD4⁺ T cells exhibited a substantially impaired ability to induce colitis, confirming the essential role of ETV1 in CD4⁺ T cell–mediated IBD pathogenesis.
At the mechanistic level, through RNA sequencing, chromatin immunoprecipitation (ChIP), and other molecular assays, the team identified SLC7A5 as a direct downstream target of ETV1. SLC7A5 is a key transporter responsible for leucine and other large neutral amino acids, and its upregulation is a hallmark of metabolic reprogramming following T-cell activation. ETV1 binds to the promoter region of the SLC7A5 gene, regulating its expression and thereby controlling amino acid uptake, as well as subsequent T-cell activation, proliferation, and Th17 differentiation.
In summary, this study systematically reveals that the ETV1–SLC7A5 axis is a critical pathway driving metabolic and functional abnormalities in pathogenic CD4⁺ T cells in IBD. Thisfinding provides anovel,promising new therapeutic target and mayfacilitate breakthrough advances in IBD treatment strategies.

