On January 14, the SACHI study led by Professor Lu Shun, academic leader of oncology at Shanghai Jiao Tong University School of Medicine Affiliated Chest Hospital and Director of the Shanghai Clinical Research Center for Lung Cancer, achieved a major breakthrough. The study was published in The Lancet. The research introduces a new precision treatment strategy—a “dual-target” regimen—for patients with advanced or metastatic non–small cell lung cancer (NSCLC) who develop resistance to EGFR-TKI therapy accompanied by MET amplification, ushering in a new era of treatment for this patient population. Professor Lu Shun served as both the first author and corresponding author of the paper, marking another Lancet publication led by his clinical research team.
The study wasa multicenter, randomized, open-label, phase III clinical trial conducted across 68 medical centers in China, enrolling 211 patients with advanced NSCLC whohad developed resistance to first-line targeted therapy and harbored MET amplification. Patients were randomly assigned to one of two groups: Combination therapy group (106 patients), receiving oral savolitinib once daily (dose adjusted according to body weight) in combination with osimertinib (80 mg); Chemotherapy group (105 patients), receiving intravenous pemetrexed plus platinum-based chemotherapy, administered in 21-day cycles. Among all enrolled patients, 137 (65%) had not previously received third-generation EGFR-TKI therapy, including 69 patients in the combination group and 68 in the chemotherapy group.
The interim analysis cutoff date was August 30, 2024. According to investigator assessments, among patients who had not previously received third-generation EGFR-TKI therapy, the median progression-free survival (PFS) was significantly longer in the combination therapy group than in the chemotherapy group (9.8 months vs. 5.4 months; HR = 0.34, 95% CI: 0.21–0.56; P < 0.0001). Consistent results were observed in the intention-to-treat population, with a median PFS of 8.2 months in the combination therapy group versus 4.5 months in the chemotherapy group (HR = 0.34, 95% CI: 0.23–0.49; P < 0.0001). Notably, even among patients who had previously received third-generation EGFR-TKI therapy, the combination therapy continued to demonstrate superiority, with a median PFS of 6.9 months compared with 3.0 months in the chemotherapy group.
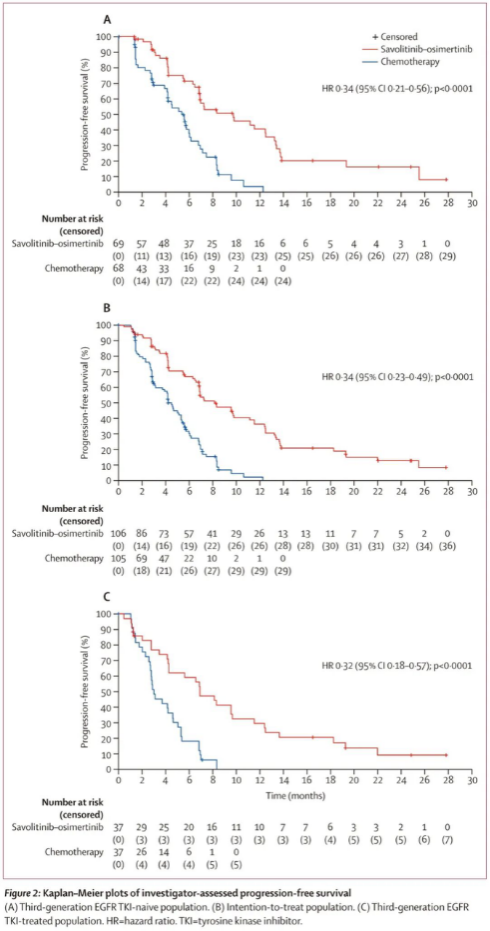
The findings confirm that in patients with EGFR-mutant advanced NSCLC who develop resistance to targeted therapy accompanied by MET amplification, the combination of savolitinib plus osimertinib offers superior efficacy compared with standard chemotherapy. Based on the results of this study, in June 2025, the National Medical Products Administration (NMPA) of China approved the combination of savolitinib and osimertinib for the treatment of patients with EGFR-mutant locally advanced or metastatic non-squamous NSCLC who have progressed after EGFR-TKI therapy and exhibit MET amplification.
This combination regimen has now become the standard treatment for patients with EGFR-mutant lung cancer who develop MET amplification after first-line targeted therapy. Beyond changing clinical practice, it has also driven further research into lung cancer targeted therapies and mechanisms of drug resistance. Most importantly, it provides a new precision treatment option for patients facing therapeutic resistance, delivering longer and better survival outcomes.

Professor Lu Shun and his team have long been committed to integrating clinical needs, translational research, and global collaboration, continuously advancing China’s participation in international oncology research. The successful completion and international publication of the SACHI study exemplify the ability of Chinese investigators to lead high-quality, multicenter clinical trials and to contribute original treatment paradigms to the global medical community.
Looking ahead, the team will continue to explore mechanisms of drug resistance, optimize combination strategies, and expand international cooperation, with the goal of delivering more precise, effective, and accessible therapies for lung cancer patients worldwide. As a representative achievement of China’s clinical research capabilities, the SACHI study stands as a compelling example of how a “China solution” can help reshape the global landscape of lung cancer treatment.

