On July 24, CAI Yujia’s team from Shanghai Jiao Tong University and Søren R Paludan’s team from Aarhus University in Denmark jointly published a groundbreaking research article in Nature (with Shanghai Jiao Tong University as the first author organization) titled “TMEFF1 is a neuron-specific restriction factor for herpes simplex virus”. They identified TMEFF1, expressed specifically in neurons of the central nervous system, as an HSV-1 restriction factor using genome-wide CRISPR screening. This research reports for the first time a neuron-specific antiviral factor independent of the interferon system, providing a new perspective for the study of the brain’s antiviral immune mechanism.
The host’s defense against microbial infections is one of the most fundamental prerequisites for species to survive, which is comparable to energy intake and life reproduction. However, immune reactions may also cause serious damage to the host and consume a significant amount of energy. Therefore, most immune mechanisms are inducible and strictly regulated. This principle is crucial for the human body to effectively combat infections, as it provides comprehensive and thorough protection for tissues highly sensitive to immune damage, like the central nervous system (CNS). Like other tissues and organs in the human body, the brain faces the risk of viral infection, and the consequence would usually be more serious. Unfortunately, we’ve got a very limited understanding of the fundamental biological question of how neurons in the brain fight against viral infections, and no neuron-specific antiviral mechanisms have been discovered yet.
Herpes simplex virus (HSV) is a widely present neurotropic double-stranded DNA virus. According to statistics, 50-80% of adults are tested positive for serology, which represents a big challenge for public health around the globe. The primary infection of HSV usually occurs in epithelial cells, and then enters sensory nerves establishing a latent infection. HSV infection may cause such symptoms as genital herpes and viral keratitis, and is also believed to be associated with Alzheimer’s disease and Parkinson’s disease. Fortunately, although most people are carriers of the herpes virus, they do not exhibit obvious symptoms of the disease. Why doesn’t HSV, as a neurotropic virus, cause brain infections in most carriers? Does the brain have a mysterious guardian? The answers to these questions remain a mystery, so it is necessary to conduct systematic research on the mechanisms by which neuronal cells combat viruses.
The research team found that depletion of TMEFF1 in stem-cell-derived human neurons led to elevated viral replication and neuronal death following HSV-1 infection. TMEFF1 mice exhibited increased susceptibility to HSV-1 infection in the brain but not in the periphery (Fig. 1). Within the brain, elevated viral load was observed specifically in neurons. The study identified TMEFF1 as a neuron-specific restriction factor essential for prevention of HSV-1 replication in the central nervous system.
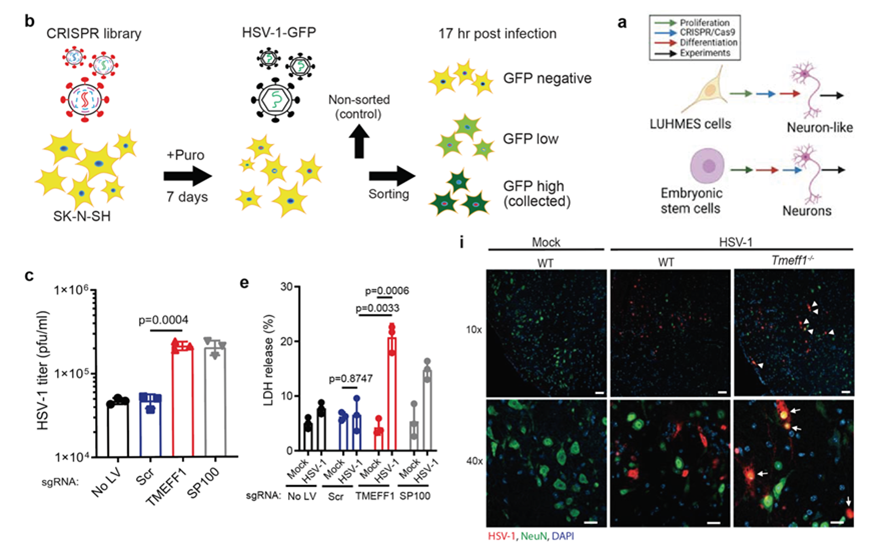
Fig. 1: CRISPR screening and in vitro and in vivo validation of the antiviral effects of TMEFF1
Further research indicated that the expression of TMEFF1 is not regulated by the classical interferon and inflammatory cytokine channels. Multiple HSV-1 restriction factors have been identified, such as MxB, IFI16, and PML nucleoprotein, which act on such processes as viral DNA nuclear entry, transcription, and DNA replication, but have one thing in common - they are all regulated by interferon, and none of them is specifically expressed in neurons. The present research reported for the first time a neuron-specific HSV-1 restriction factor independent of the interferon system. The research also found that TMEFF1 inhibited virus entry into the membrane by interacting with the virus’ entry receptor Nectin-1 and non-muscle myosin heavy chain IIA/B. It simultaneously interfered with the virus binding phase mediated by HSV-1 gD protein and the virus fusion phase mediated by HSV-1gB protein, thereby restricting virus replication (Fig. 2). This dual defense mechanism reflects the crucial importance of blocking HSV-1, a highly prevalent virus, outside the central nervous system for maintaining host health and function.
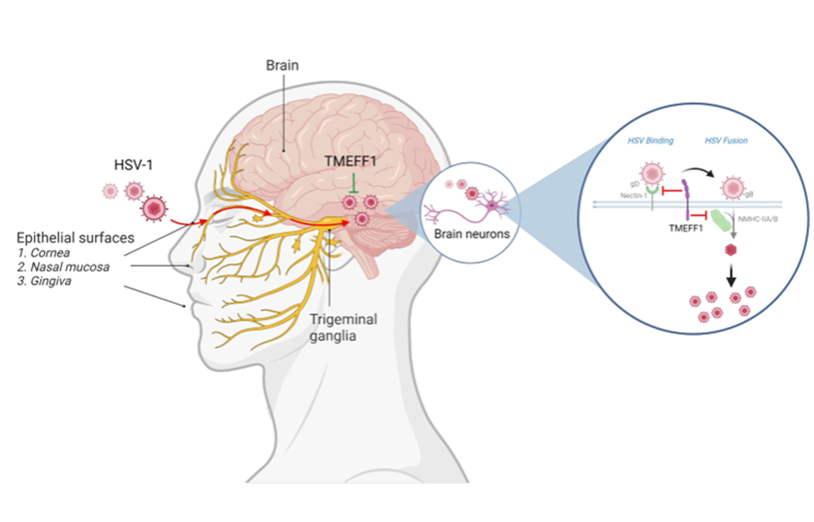
Fig. 2: TMEFF1 restricting HSV-1 in the CNS neurons
The anti-infective immune responses need to find a delicate balance between eliminating pathogens and avoiding immune-mediated damage. Especially for highly sensitive parts like the brain, uncontrolled innate and adaptive immune responses may lead to devastating consequences. Not only did the research reveal for the first time a neuron-specific immune mechanism, but it also provided important clues for understanding the optimal antiviral immune mechanism in the brain. Researchers suggest that the optimal antiviral immune mechanism in the brain is the combination of innate immunity and interferon-driven immunity. Innate immunity responds immediately, establish and increase the threshold of infection, while interferon-driven immunity is more powerful but may lead to tissue damage. Their combination, however, provides a balance that minimizes the damage of viral infections to the central nervous system.
In the research, based on the antiviral mechanism of TMEFF1, a peptide derived from TMEFF1 was found to deliver high efficacy in resisting HSV infection. These findings provide new targets for the development of novel antiviral therapies. This is expected to help with the development of new HSV drugs and have significant implications for public health and medical research.
Prof. CAI Yujia from the Shanghai Center for Systems Biomedicine, Shanghai Jiao Tong University and Prof. Søren R Paludan from Aarhus University are the co-corresponding authors of the academic article, and Dr. DAI Yao from Prof. CAI Yujia’s research ream at the Shanghai Center for Systems Biomedicine, Shanghai Jiao Tong University and Dr. Manja Idorn from Prof. Søren R Paludan’s research team at Aarhus University are the co-first authors. Professors PAN Xiaoyong, ZHU Jinwei, DA Lintai, and LU Qing from Shanghai Jiao Tong University, as well as JIANG Zhuofan, ZHONG Yiye, and ZHANG Shuhui from Prof. CAI Yujia’s research team, made important contributions to the successful completion of the research. It was greatly supported by two general projects of the National Natural Science Foundation of China (Grant No. 31971364;32370148).
The research was inspired by a rather casual conversation in 2016, when Dr. CAI Yujia working in Denmark for research on viral vectors and gene editing went to visit Prof. Søren who was engaged in immune research. CAI proposed that “since there are so many things unknown between the virus and the host, why don’t use the gene editing and screening tools to systematically examine it all? There will definitely be new discoveries!” Søren answered straightforwardly, “I happen to have a lot of HSV here”. Both parties hit it off immediately. Although the research paper was successfully published, the research process was full of hardships and challenges, spanning 8 years. Dr. CAI Yujia overcame loads of difficulties including insufficient funding and personnel in the early stage, and finally locked in the target molecule TMEFF1 two years later, and then the research was focused on its molecular mechanism. Curiosity about pure science has, as always, been the strongest driving force behind the continuous progress of CAI’s team. The research had gone through a long cycle, a lot of challenges, cross-border coordination among China, Denmark and USA, collaboration among multiple teams around the world, and interdisciplinary communications, to solve the unique antiviral mystery of the brain and answer one of the most fundamental biological questions about how the brain fights against viral infections.
Comments by Experts

ZHAO Guoping
(Academician, Chinese Academy of Sciences, and director of the CAS Key Laboratory of Synthetic Biology)
The research by CAI, Søren et al. discovered and identified a previously undiscovered viral restriction factor TMEFF1 that is neuron-specific. This factor inhibits virus entry into cells through a double safety mechanism that binds to the herpes simplex virus (HSV) receptor Nectin-1 and non-muscle myosin chain (NMHC) IIA/B. That is, it exerts a restricting effect at the first moment of virus infection, or at the viral membrane entry stage. TMEFF1 differentiates itself from most known restriction factors as, independently of the regulation by the classical innate immune response and interferon (IFN) system, it functions through an intrinsic, non-inducible mechanism of action, which is beneficial for neurons to respond quickly to HSV virus infection and is of great significance for maintaining the health of the brain and neurons. Whereas there are billions of people infected with the herpes simplex virus worldwide, the TMEFF1 research has significant implications for public health around the globe.
The research team successfully identified the antiviral factor TMEFF1 using genome-wide CRISPR screening, implying the potential applications of gene editing in virological research. In addition, the researchers discovered an antiviral peptide based on the antiviral mechanism of TMEFF1, which offers great clinical application prospects as well as an important direction for antiviral drug development in the future.
The discovery of TMEFF1 enriches our understanding of the antiviral mechanism in the brain, enabling us to comprehend the “delicate balance” that has evolved in the brain between eliminating viral infections and avoiding immune cell damage. It also provides a new scientific rationale for the development of preventive and therapeutic measures for HSV in the future, and this is of great significance in both scientific and translational areas. Not only does the research represent a major breakthrough in virology, but it is also an important intersection of neuroscience and immunology, making an important contribution to human health.

LI Bin
(Distinguished professor of Shanghai Jiao Tong University, and associate director of Shanghai Institute of Immunology)
Herpes simplex virus 1 (HSV-1) is a highly prevalent neurotropic double-stranded DNA virus, infecting approximately 50-80% of adults serologically. HSV-1 infection may lead to oral herpes or blinding keratitis. In special cases, HSV-1 infection may enter the central nervous system from the peripheral nervous system, causing herpes simplex encephalitis with a mortality rate of up to 70%. Even if a patient is cured, he or she may still face a high degree of neurological sequelae in the later stage. The central nervous system, as one of the most important and sensitive tissues in the human body, is strictly protected by the human systems, like the blood-brain barrier that filters out some virus invasions. Various indications suggest that neurons may have their unique antiviral strategies for directly restricting virus replication without activating inflammatory responses. It is, however, still unclear what mechanisms neurons use to exert their unique antiviral functions.
In the present research, CAI and other researchers reported for the first time the neuron-specific HSV restriction factor TMEFF1 using genome-wide CRISPR screening. And they confirmed the efficacy of TMEFF1 in restricting viral infection in human neurons derived from stem cells and TMEFF1 mice, elucidating its specific molecular mechanism of action. TMEFF1 directly blocked the process of virus entry into cells by interacting with cell surface receptors that mediated virus binding and fusion, thus effectively blocking the virus from entering neuronal cells in the first place. Interestingly, compared to various interferon (IFN) - mediated viral restriction factors, the expression of TMEFF1 is not regulated by IFN or inflammatory cytokines, thus avoiding pathological damage caused by excessive IFN. The significance of the research lies in the first identification of TMEFF1 as a neuron-specific HSV restriction factor and the discovery of its protective mechanism against viral infections in neurons. The research findings have significant implications for the study and understanding of the human body’s response to viruses.
About the Team
CAI Yujia’s research team has long been committed to identifying drug targets, developing gene therapy tools, and researching on translational medicine, and has made significant achievements in target discovery, vector development, and clinical research. To be more exact, they have developed a virus-like particle VLP, and a DC-targeted VLP mRNA vaccine technology, and identified the new antiviral factors TMEFF1 and PARP1. Among them, VLP has been used in clinical studies of the gene editing therapy for two diseases. In recent years, they have, as the corresponding authors, published multiple academic papers in Nature (2024), Nature Biotechnology (2021), Nature BME (2021/2024), Cell Stem Cell (2024), and other journals. The research team has a strong interest in the development of new gene editing and delivery tools, new targets for tumors and aging, innovative therapies for difficult to treat diseases, intersection of artificial intelligence and biomedicine, and fundamental issues in life sciences. Outstanding students and postdoctors with relevant research backgrounds are welcome to join the team and work together to improve the quality and length of human life with science and technology.

