A collaborative team led by Dr. Svetoslav Chakarov from the College of Basic Medical Sciences, Shanghai Jiao Tong University School of Medicine, together with national and international partners, has published a landmark Resource paper in Nature Immunology entitled “Temporal and spatial atlas of eosinophil specialization across tissues.”
The study establishes time of tissue residency as a core principle governing eosinophil maturation, diversity, and function, fundamentally reshaping how eosinophil biology is understood across organs. To uncover this principle, the authors generated the first integrated single-cell transcriptomic and proteomic atlas of eosinophils across multiple tissues, providing an unprecedented view of eosinophil life history in vivo.
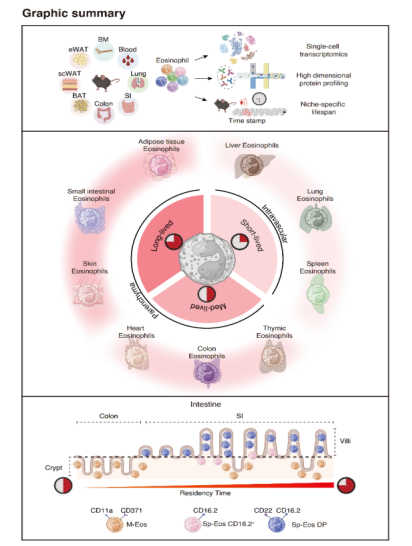
Eosinophils are multifunctional granulocytes implicated in immunity, metabolism, and tissue repair, yet the rules governing their specialization have remained unclear. This study demonstrates that how long eosinophils persist within a tissue niche is a dominant determinant of their functional diversification, acting in concert with local environmental cues. By combining single-cell RNA sequencing, high-dimensional surface proteomics, and inducible fate mapping, the authors show that eosinophils follow a conserved differentiation continuum from bone marrow to blood and into tissues, where extended residency enables progressive specialization. Short-lived eosinophils remain transcriptionally uniform, whereas long-lived eosinophils acquire layered molecular and phenotypic identities.
To systematically define residency-linked specialization, the authors constructed the first multi-organ single-cell and proteomic atlas of eosinophils, profiling cells from bone marrow, blood, lung, small intestine, colon, skin, and multiple adipose depots. This atlas reveals that eosinophils adopt tissue-imprinted transcriptional and surface protein signatures, but crucially, the degree of heterogeneity scales with lifespan. The work also delivers validated marker panels that enable prospective isolation of eosinophil subsets across tissues. Additionally, the research team established a rigorously validated biomarker panel and identified a series of markers associated with differentiation trajectories—including CD371, CD11a, CD22, and CD16.2—that distinguish progenitor, circulating, and specialized tissue-resident eosinophils. This work advances eosinophils from an underrepresented cell type into a tractable system for single-cell analysis.
The study uncovers striking contrasts between tissues with different residency dynamics:
Lung eosinophils are short-lived, largely intravascular, and transcriptionally uniform. Colon eosinophils exhibit intermediate residency with limited diversification. Small intestinal eosinophils are long-lived, parenchymal, and diversify into multiple transcriptionally and phenotypically distinct subsets. Using fate mapping, the authors directly link eosinophil lifespan to the emergence of specialized states, demonstrating that extended tissue retention is required for eosinophil diversification.
Residency-driven specialization is further sharpened by spatial organization. Within the small intestine, long-lived eosinophils preferentially localize to villi, while short-lived populations remain confined to crypts. Along the intestinal axis, the loss of villi coincides with reduced eosinophil lifespan and diminished heterogeneity in the colon. These findings show that microanatomical localization and residency time act together to instruct eosinophil fate, extending niche-based principles of immune regulation to granulocytes.
This work positions time of tissue residency as a central axis of eosinophil specialization, analogous to emerging concepts in macrophage and neutrophil biology. By delivering the first cross-tissue single-cell and proteomic eosinophil atlas and uncovering residency time as a governing principle, the study provides a foundational resource for investigating eosinophil roles in barrier immunity, metabolism, inflammation, and tissue repair. An interactive public portal accompanies the study, enabling broad community access to this resource.

