Guan's research found that both immature and mature neutrophils enter tumors and undergo epigenomic, transcriptomic and InfinityFlow differentiation pathways through single-cell RNA sequencing. Single-cell RNA sequencing, InfinityFlow and other methods were used to analyze the differentiation pathway of neutrophils within tumors. It was found that both immature and mature neutrophils enter tumors and undergo reprogramming at the epigenomic, transcriptomic and proteomic levels, and are transformed into T3 terminally differentiated neutrophils with high expression of dcTRAIL-R1, and then localize in the core area of tumor tissues, which are characterized by high glycolysis and hypoxia, to play the role of pro-angiogenic and pro-cyclic functions. The neutrophils were then localized in the core region of tumor tissues with high glycolysis and hypoxia, and played a pro-angiogenic role to support tumor growth. This study not only analyzed the specific differentiation pathways of tumor neutrophils, but also identified surface markers that can distinguish tumor neutrophils of different pathways of differentiation, which is expected to provide new targets and new ideas for tumor immunotherapy targeting neutrophils.
Neutrophils are rapidly recruited into damaged tissues and play a key immunoregulatory function in the body's immune response to external infections and injuries. In pathological conditions such as cancer, the degree of neutrophil infiltration in tumor tissues is strongly associated with immunotherapy resistance and poor prognosis of patients. The traditional view of intra-tumoral neutrophils is that they are a subpopulation of cells with immunosuppressive properties and are referred to as granulocyte-myeloid-derived suppressor cells (MDSC). With the in-depth study of intra-tumor neutrophils at multi-dimensional levels such as density, maturity, surface markers, and transcriptome, intra-tumor neutrophils exhibit complex features and functions; however, the pathway of differentiation and development of intra-tumor neutrophils and how they can be uniformly remodeled to pro-tumorigenic functions are still unknown, which has greatly limited the research and development of neutrophil-based tumor immunotherapeutic targets. Therefore, this study represents a major breakthrough in the field of neutrophil research.
To investigate the heterogeneity of neutrophils in tumors, the research team comprehensively analyzed neutrophils in a mouse model of pancreatic ductal adenocarcinoma (PDAC) by single-cell RNA sequencing (scRNAseq), and identified the presence of three different differentiated states of T1, T2 and T3 neutrophil populations in the tumor microenvironment. populations in the tumor microenvironment, which are distinct from bone marrow, spleen and peripheral blood neutrophils at the transcriptome level. By assessing the nuclear morphology and maturation status, T1 and T2 were identified as transitional populations derived from immature and mature neutrophils, respectively, and T1 and T2 developed into terminally differentiated T3 neutrophils after further reprogramming. In addition, ATACseq revealed that both immature and mature neutrophils within the tumor carried specific chromatin accessibility features and transcription factor activities of the T3 cell population, suggesting that neutrophils entering the tumor tissue have the ability to initiate reprogramming regardless of their maturity.
Then the research team identified dcTRAIL-R1, a surface marker that can be used to distinguish tumor reprogrammed T3 neutrophils, with the help of InfinityFlow technology, and combined it with CD101, a surface marker that can distinguish immature and mature neutrophils[5], to classify tumor neutrophils into dcTRAIL-R1-CD101- (T1 immature), dcTRAIL-R1-CD101+ (T2 mature) and dcTRAIL-R1+CD101-/+ (T3 immature/mature), and validated this method of tumor neutrophil classification at the transcriptome level. Subsequently, the research team tracked the generation of tumor reprogrammed T3 neutrophil populations and their dynamic patterns of change in vitro and in vivo, and found that neutrophils were able to up-regulate their dcTRAIL-R1 expression upon entering the tumor microenvironment or after treatment with tumor culture supernatants, accompanied by elevated expression of T3-specific gene features. With the help of BrdU pulse labeling method, the research team found that the half-life of neutrophils in tumors was significantly increased compared with that of peripheral neutrophils, and the dcTRAIL-R1 level of neutrophils gradually increased after entering tumors and could be maintained for at least 5 days, which indicated that the T3 phenotype was closely related to the prolongation of the lifespan of neutrophils in tumors.
Finally, the team investigated the localization characteristics of T3 neutrophils within tumors and their role in tumorigenesis, and found that they are primarily located in a unique ecological niche within tumors characterized by hypoxia and hyperglycolysis, and that they express high levels of vascular endothelial growth factor α (VEGFα) and induce vascular remodeling in the core region of tumors. Co-injection of T3 neutrophils with tumor cells significantly accelerated tumor growth, and conversely, removal of T3 neutrophils with anti-dcTRAIL-R1 antibody or inhibition of their function with anti-VEGFα antibody eliminated the tumor growth-promoting effect of T3 neutrophils. In addition, the team analyzed publicly available single-cell data and found that multiple neutrophil populations were reprogrammed to T3 neutrophil populations in a variety of mouse tumor models and clinical PDAC patients, and found a significant correlation between the gene expression profile of T3 neutrophils and poor prognosis in cancer patients, further suggesting the role of T3 neutrophil populations in promoting tumor growth.
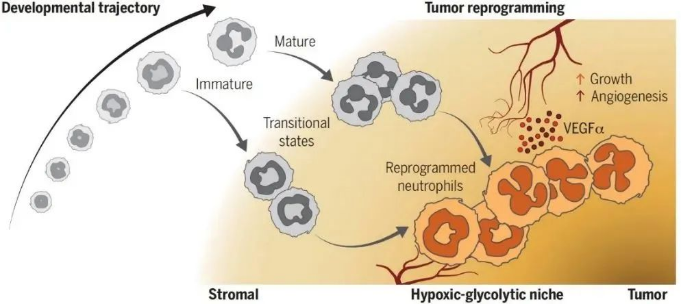
▲ Fig. Intratumoral neutrophils undergo a common differentiation pathway to reprogram into pro-angiogenic neutrophils that support tumor growth
When entering the tumor tissue, immature and mature neutrophils differentiate into T1 and T2 transitional cell populations, respectively, which are further reprogrammed to fuse into terminally differentiated T3 neutrophils, which express high levels of VEGFα and are localized in a hypoxic, hyperglycolytic ecological niche close to the core of the tumor, maximizing their pro-angiogenic effects and thus tumor growth. growth.
Taken together, Guan's team revealed that neutrophils in the tumor microenvironment, whether mature or immature, have the ability to remodel in response to environmental signals and follow a common differentiation pathway to converge into an end-state T3 tumor-promoting phenotype. In homeostasis, the ability of neutrophils to remodel in response to tissue signals facilitates tissue homeostasis regulation and immune repair. In contrast, in organismal pathological conditions such as within tumors, this reprogramming capacity of neutrophils instead generates large numbers of pro-angiogenic T3 neutrophils that support tumor growth. This study demonstrates that neutrophils, despite their short lifespan, have the ability to adapt their functions to different tissue microenvironments by modulating their functions according to different tissue signals. This research may provide the basis for new therapies targeting neutrophils that may be effective not only in cancer but also in other neutrophil-related diseases. In addition, the findings provide new insights into the function of neutrophils.

This work began 4 years ago and was completed by members of the Myeloid Cell Biology Laboratory team. Postdoctoral fellows Sufen Huang, Yongheng Guo, Deli Chen and Associate Researcher Changming Shi of Affiliated Renji Hospital of Shanghai Jiao Tong University School of Medicine are the co-first authors of this paper, the first and co-corresponding authors are the postdoctoral fellows ofGuan 's laboratory, andNg Lai Guan at Renji Hospital-Shanghai Institute of Innovation in Immunotherapy of Shanghai Jiao Tong University School of Medicine is the main corresponding author of this paper.
The research team said the study received strong support from Shanghai Jiao Tong University School of Medicine and Renji Hospital, especially in terms of clinical resources and professional support.

