CTLA-4 blockade antibody enhances effector T cell function and mediates intratumoral Treg cell depletion to induce durable antitumor immune responses and tumor regression. However, the responsiveness of CTLA-4 blockade therapy remains low clinically and severe immunotherapy-related adverse effects are associated with CTLA-4 monotherapy.
Lactate accumulation has been regarded as a marker of metabolically abnormal tumor microenvironment, which exhibits important regulatory role in the maintenance of tumor-infiltrating Treg cell function. It has been demonstrated that lactate is involved in the regulation of the PD-1 monotherapy efficacy, whether it modulates CTLA-4 expression in Treg cells to affect the CTLA-4 blockade therapyresponses is worth exploring.
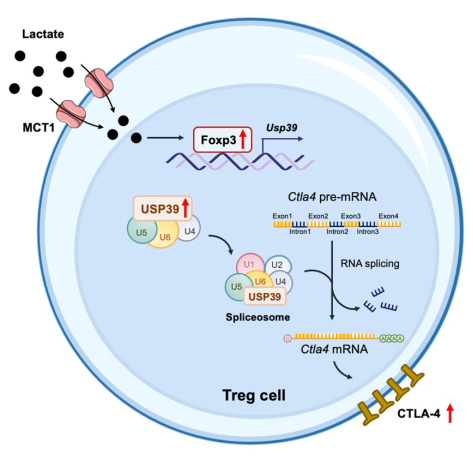
The research team constructed murine tumor models to address whetherlactate uptakeby Treg cells affects CTLA-4 blockade therapy.Tumor bearing mice with Treg cellconditional knockout ofSlc16a1, which encodes lactate transporter MCT1, cannot respond to CTLA-4 blockade therapy. Mechanistically, lactate uptake by Treg cells increased Foxp3 expression. And then Foxp3 promoted the transcription ofUsp39, a component of RNA splicing machinery, thereby sustaining USP39-mediated RNA splicing to facilitate CTLA-4 expression, which in turn contributes to the efficacy of CTLA-4 blockade. Moreover, the efficiency of CTLA-4 RNA splicing was increased in tumor-infiltrating Treg cells from patients with colorectal cancer. These findings highlight USP39 as a lactate sensor for Treg cell environmental adaptation and identify a mechanistic connection between lactate and RNA splicing in Treg cells, with therapeutic implications for antitumor immunity.
Ph.D student Rui Ding, associateresearchfellow Xiaoyan Yu of Shanghai Institute of Immunology, SJTUSM,and Professor Zhilin Hu of Nanjing Medical University are the co-first authors of this paper. PI Qiang Zou and Youqiong Ye of Shanghai Institute of Immunology, SJTUSM, Professor Ren Zhao of Ruijin hospital, SJTUSM and Professor Zhi-yu Ni of Hebei University of Engineering are the co-corresponding authors of this paper.

