On March 28th,2023,the team of Academician Xianqun Fan from the Department of Ophthalmology atShanghaiNinth People's Hospital, Shanghai Jiao Tong University School of Medicine published a research article titled "Nuclear PD-L1 promotes EGR1-mediated angiogenesis and accelerates tumorigenesis" in Cell Discovery. The study reveals a higher proportion of nuclear programmed cell death ligand 1 (PD-L1) in uveal melanoma (UM) cells, which promotes tumor angiogenesis through transcriptional activation of pro-angiogenic factor early growth response factor 1 (EGR1). Additionally,the study proposes a potential treatment option for UM patients, involving a combination of anti-PD-L1 immunotherapy and HDAC2 inhibitors.

Tumor immunotherapy, the third revolution in cancer treatment following chemo-radiation and targeted therapies, has been extensively investigated in recent years. Among various immunosuppressive molecules, PD-L1 plays a pivotal role in tumor immune evasion. PD-L1 expressed on the surface of tumor cells interacts with programmed cell death receptor 1 (PD-1) on the surface of T cells in the tumor microenvironment. This interaction inhibits T cell proliferation and activation, resulting in immune escape, tumor growth, and metastasis. Despite the remarkable success of anti-PD-1/PD-L1 immunotherapy in treatingvarious cancers, its efficacy in UM remains dissatisfactory. In the largest multicenter clinical trial conducted to date, only 3.6% of patients with metastatic UM treated with anti-PD-1 or anti-PD-L1 antibodies exhibited treatment response. These patients had a median progression-free survival and overall survival of 2.8 and 7.6 months, respectively, with no observed survival benefit.
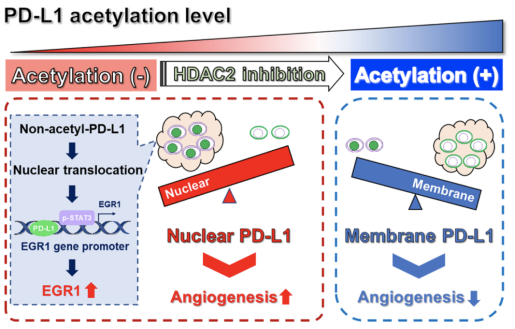
Recent studies have revealed that PD-L1 can be translocated into the nucleus and play a non-immune regulatory role in promoting cancer.For example, histone deacetylase 2 (HDAC2)-dependent deacetylation of membrane PD-L1 promotes its nuclear translocation, regulating the immune response in breast cancer. This finding underscores the potential importance of nuclear PD-L1 intumorigenesis and its potential role in resistanceof anti-PD-1/PD-L1 immunotherapy. However, the distribution pattern and function of PD-L1 in UM are still poorly understood.
The team led by Academician Xianqun Fan in the Department of Ophthalmology at Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine has been devoted to the clinical treatment and basic research of UM for many years. Based on clinical sample resources,they first discovered an elevated expression level of nuclear PD-L1 in UM, which was associated with poor prognosis. Further, they revealed that nuclear PD-L1 had no significant effect on the proliferation and migration ability of UM cells, but markedly increased their pro-angiogenic ability. Through combined CUT&Tag and RNA-seq analysis,they found that nuclear PD-L1 could transcriptionally activate of the expression of the pro-angiogenic factor EGR1. Mechanistic studies demonstrated that nuclear PD-L1 promoted the binding of p-STAT3 to the EGR1 promoter region, thereby activating EGR1 expression. The use of HDAC2 inhibitors could restore the acetylation level of PD-L1 in UM and prevent its nuclear translocation.Thus, it decreased EGR1 expression levels, which would inhibit UM angiogenesis. Therefore, the combination ofanti-PD-L1 immunotherapy and HDAC2 inhibitors represents a potential treatment strategy for UM patients.
Dr. Jie Yu, Ai Zhuang and Xiang Gu are the co-first authors of the paper. Academician Xianqun Fan, Professor Renbing Jia and Dr. Peiwei Chai are the corresponding authors. Professor Shengfang Ge,Dr. Jing Ruan,Dr. Yu Hua andDr. Ludi Yang are co-authors. The research was supported by grants from the National Natural Science Foundation of Chinaandthe Science and Technology Commission of Shanghai.

