Researchers from the laboratory of Leng-Siew Yeap at the Shanghai Institute of Immunology, Shanghai Jiao Tong University School of Medicine and Fei-Long Meng at the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences have reported the results of their groundbreaking study to elucidate the mechanisms that promote somatic hypermutation at the antigen-binding sites of antibody genes. These findings resolve a long-standing question that has puzzled antibody researchers for more than 40 years and provide new insights into the development of the next-generation humanized antibody animal models.
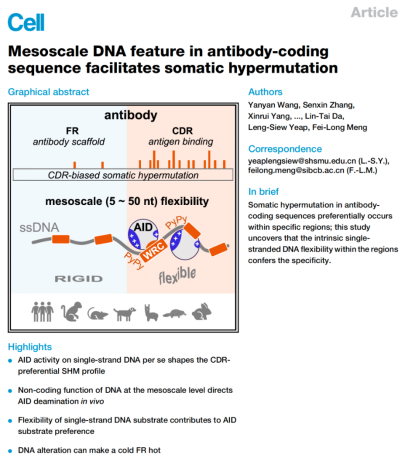
One of the long-standing questions puzzling antibody researchers is why somatic hypermutations are concentrated in the three short, non-consecutive complementarity determining regions (CDRs) in the antibody gene sequence. First documented by the labs of David Baltimore, Klaus Rajewsky, and Leroy Hood in two Cell papers in the early 1980s, CDR hypermutation is well accepted and taken for granted by immunologists as an axiom in textbooks, although the mechanism is unknown. The study, published online on April 24, 2023 in Cell, reports that the flexible single-stranded DNA feature is the key to CDR-hypermutation. Using a cutting-edge, high-throughput biochemical assay that can test many DNA substrates for their deamination/mutation ability, the researchers found that the CDR hypermutation is evolutionarily conserved in species that use somatic hypermutation to diversify their antibody repertoire. Using the powerful passenger antibody gene allele mouse model system, which allows the detection of unselected mutational events, and the CRISPR/Cas9 system, which allows rapid generation of mice with DNA sequence alterations, the researchers found that the CDR hypermutability depends on the DNA sequence context at the mesoscale level (5-50 bp). Using a combination of molecular dynamics simulations and single-molecule biochemistry, the researchers demonstrated that the targeting preference of the DNA mutator enzyme, activation induced cytidine deaminase (AID), is directly regulated by the flexibility of the single-stranded DNA substrate. Analysis of the antibody gene sequence showed that DNA sequences encoding the CDRs have evolved highly flexible properties to facilitate hypermutation, revealing a non-coding role of these sequences, and explaining the hypermutation pattern in lymphoma. Finally, regions that are normally “cold” for hypermutation can be made “hot” by engineering flexible DNA sequences in the “cold” regions, opening the door to the next generation of humanized animal models for antibody discovery.
The first author of this paper is Yanyan Wang, a research assistant who initiated this project and made the initial discoveries in mouse models in the Yeap lab, and continued to work on this exciting project as a Ph.D. student in the Meng lab. We would like to thank all of our wonderful collaborators on this study, Dr. Frederick Alt at Harvard Medical School, Dr. Qiang Pan-Hammarström at Karolinska Institute, Dr Lin-Tai Da at Shanghai Jiao Tong University, Dr. Xiaoqi Zheng at Shanghai Jiao Tong University School of Medicine, Dr. Yaofeng Zhao at China Agricultural University, Dr. Jie Song at Hangzhou Institute of Medicine, Dr. Shaohui Huang at University of Chinese Academy of Sciences, Dr. Zhiwei Cao at Fudan University and Dr Jiaquan Liu at Shanghai Institute of Biochemistry and Cell Biology. This work was supported by National Natural Science Foundation of China, National Key R&D Program of China, and etc. The authors also acknowledge the support of the Center of Immune-Related Diseases at Shanghai Institute of Immunology and Core Facilities at the Shanghai Institute of Immunology and the School of Basic Medical Science.
The Yeap lab welcomes enthusiastic students and postdoctoral fellows to join the group. Interested individuals can contact Dr Yeap at yeaplengsiew@shsmu.edu.cn

Yanyan Wang’s poster presentation at SII-CIML symposia, 2018.
First from left: Dr Leng-Siew Yeap, second from right: Yanyan Wang.

