Biomedical imaging plays crucial roles in cancer diagnosis, intraoperativenavigation, and postoperative assessment. Fluorescence imaging has attracted widespread attention due to its low cost, the ability to offer fast feedback, high sensitivity, and low level of hazardous radiation. There has been an increasing use of biomedical imaging in biomedical research.
Conventional optical imaging uses visible light as excitation light. But due to tissue absorption and light scattering, excitation light is greatly attenuated when passing through biological tissues. Excitation light in the NIR range (650-900 nm) can achieve deeper tissue penetration and lower tissue spontaneous fluorescence, thereby realizing deep tissue imaging with higher signal-to-background ratio (SBR).
FDA approved the use of Cytalux (pafolacianine sodium, previously known as OTL38) in 2021 in adult ovarian cancer patients to enhance surgeons' ability to visualize lesions as they operate. As a near-infrared diagnostic reagent, Cytalux is administered preoperatively by intravenous injection. Ovarian cancer is usually associated with an overexpression of folate receptors in the cell membrane. Cytalux binds to the folate receptors specifically, emitting light under near-infrared irradiation, thereby boosting precise localization and resection of the lesions.In light of these potentials, tumor-targeting contrast agents for near-infrared fluorescent imaging have become emerging hotspots in novel drug research and development.

Recently, the research group led by Zhong Qing and Wang Weiwei from Shanghai Jiao Tong University School of Medicine worked jointly with the team led by Xu Bin and Chen Qi from Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine and that by Xu Lu from Ruijin Hospital, Shanghai JiaoTong University School of Medicine. Theyjointlypublished an article titled "A Novel Spraying Nanoprobe for Renal Cell Carcinoma in Humans" inLife Medicine. A simple, highly efficient, and ingeniously designed tumor imaging approach is described. A nanoparticle could be directly applied to renal tumor tissues by spraying, without the need for an intravenous injection, to enable real-time tumor imaging.
In their article, a near-infrared hypoxia-targeting dye and an anionic surfactant (surfactin) were self-assembled into S-4-NP nanoparticles in aqueous solution. The fluorescence of the dye was self-quenched due to close proximity as a result of the particles' nanostructure. When the nanoparticles came into contact with cancer cells, the dye specifically recognized the organic anion-transporting polypeptides (OATPs) on the cell membrane, was released from the nanoparticle structure, and accurately entered the cell. Inside the cell, the fluorescence of the free dye was restored, thus marking cancer cells with a NIR signal (Fig. 1).
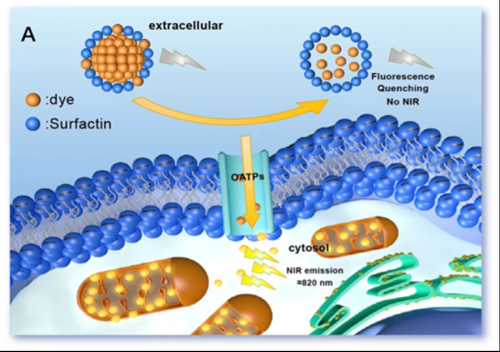
Fig. 1 Validation of the xenograft mouse model
Tumors were first removed from the xenograft model from the mice, and the solution with compounds was then immediately sprayed onto the cancerous tissue. NIR images were obtained at 3, 6, 9, and 12 min after spraying. There was an obvious increase in the uptake value (> 2-fold) from 3 min to 12 min, indicating a higher stability of these nanoparticles.
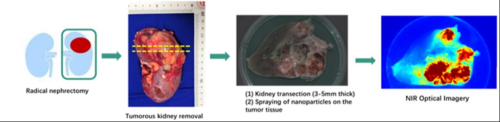
Fig. 2 Validation of radical nephrectomy
Their study further investigated the imaging properties of S-4-NP during radical nephrectomy for the purpose of academic research (Fig. 2).
Nephron-sparing surgery (NSS) is the most important surgery to treat renal cell carcinoma (RCC), which preserves renal function maximally while offering radical treatment of tumors. However, positive surgical margins (PSMs) after NSS cannot be completely avoided, resulting in local recurrence and reduced survival. There is still a lack of fast responsive, non-toxic tumor-specific contrast agents for PSM detection in clinic.
During surgery, S-4-NP can be directly sprayed onto the surface of ex-vivo cancerous tissues, followed by near-infrared imaging to visualize the marked cancerous tissues in 3 min. Using S-4-NP, surgeons can quickly localize the boundaries between cancerous and normal tissues and assess surgical margins (for full video of the imaging procedure, see Fig. 3).
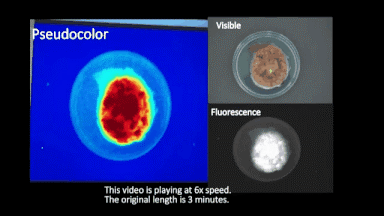
Fig. 3
NSS is the preferred surgical approach for early renal tumors, though PSM remains an unresolvedissue. Conventionally,surgens judge the nature of surgical margins using naked eyes duringthe operation. Confirmation of suspicious sites requires preparation of intraoperative frozen sections or postoperative paraffin-embedded sections,either ofwhich is time-consuming. The near-infrared fluorescent imaging technique described in their study is fast and highly efficient and enables surgeons to adopt any remedial measures neededupon finding of PSMs. This technique saves surgical time, improves prognosis, reduces tumor recurrence, and is worthy of being translated to the clinic.

