STING (Stimulator of interferon genes) is a vital regulating molecule in the process of natural immunization. The cGAS-STING pathway can sense free DNA and activate the downstream NF-κB and IRF3 signal molecule, and produce Type I interferon, which plays a vital role to the process of infection, autoimmune disease, inflammation response, cell aging, etc. The researches in recent years also suggest that STING has mediated the process of non-classical autophagy induced by free DNA or cGMAP, which often doesn’t warrant the involvement of classical autophagic molecules such as ULK 1 compounds or VPS34-Beclin1 compounds. However, at present, it remains unclear as regard to STING’s function in energy deficiency-induced classical autophagic pathways.
On May 5, the research paper titled STING controls energy stress-induced autophagy and energy metabolism via STX17 is published on the Journal of Cell Biology, an internationally renowned journal by Zhong Qing and Liu Xiaoxia research group with Department of Physiology and Pathology of SJTUSM, in conjunction with Rong Yueguang research group with Department of Pathogen Biology of Tongji Medical College of Huazhong University of Science and Technology, and Helmut Kramer Team with Southwestern Medical Center of University of Texas.
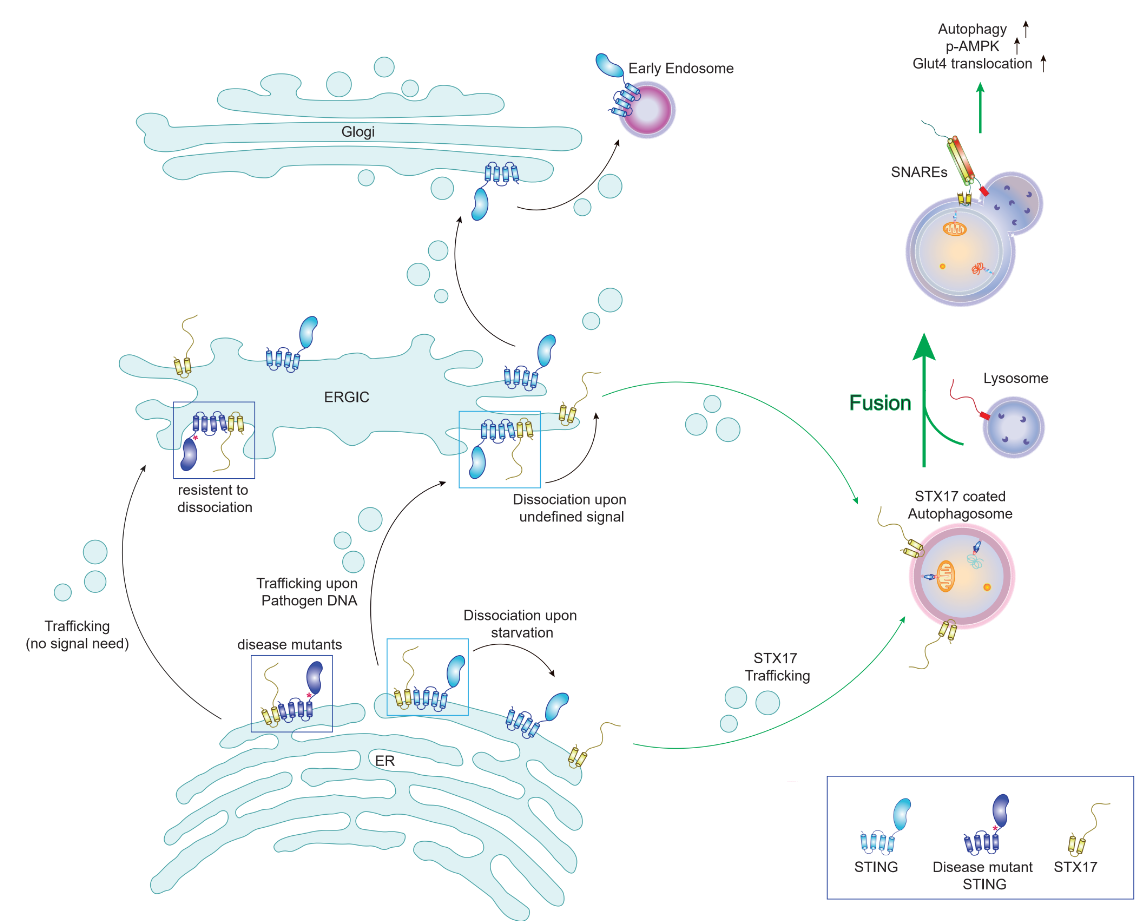
In this research, the researchers, using fruit fly, mouse and cultured cell system, reveal that the activation of STING molecules can negatively regulate the autophagic process as caused by deficiency of energy, and the mechanism lies in: STING can, via binding with SIX17 proteins vital for autophagic membrane fusion, impact on STX17 protein’s process of transport from endoplasmic reticulum to complete autophagosome, consequentially inhibiting the autophagosome-lysosome fusion efficiency and lowering the autophagy. This research offers a new approach for revealing the non-immune function of STING molecules, as well as its relationship between autophagy and energy metabolism.

First of all, the research group finds, through analyzing on autophagic phenotype in fruit fly’s fat body cells deficient of STING that, deficiency of STING can result in rising autophagic flux and accelerated fusion between autophagosome and lysosome, and infers through the double knockout experiment of autophagic membrane’s fusion with key molecule STX17 that, it is highly possible that the rising autophagic flux induced by deficiency of STING may have been exercised through STX 17.
Subsequently, using mouse exercise model the researchers attest again that,the mice knocked out of STING witness rise in autophagic level induced by acute exercise, and intensified degradation of autophagic substrates, and that, there is a rising level of activation of AMPK (Adenosine 5‘-monophosphate (AMP)-activated protein kinase), a key molecule for energy metabolism induced by exercise.
It is proved through further analysis on activation of AMPK molecule substrate and testing on metabolic indices of sugar and fat that, deficiency of STING may, via up-regulating exercise to stimulate AMPK activity in lower skeletal muscle, consequentially increase the level of phosphorylation of its downstream TBC1D1(TBC1 Domain Family Member 1)molecule as well as the course of transport of its regulated GLUT4(Glucose transporter type 4)to the plasma membrane of the skeletal muscle, which contributes to significantly increased intake of the glucose molecules in blood by the STING knockout mouse’s skeletal muscle cells, as well as to strengthened athletic ability. Deficiency of STING does not, however, impact on the level of the fat metabolism inside the mouse in acute exercise model. The experiment of this part puts forward creatively the STING’s effects on autophagy’s negative regulation function and the organism’s energy metabolism. Well then, how does STING, on the part of molecular mechanism, act on the autophagic process? Coming next, the researchers find through researches on cells deficient of STING that, deficiency of STING can accelerate the fusion process of autophagosome and lysosome in the autophagy induced by deficiency of energy, and moreover, this function is dependant upon the regulating protein of classical autophagy pathways, e.g. STX17, ULK1/2, ATG14, ATG9A, ATG5, etc.
Further researches on the molecular mechanism show that, the STING molecules residing in endoplasmic reticulum can have direct bonding with STX 17 protein, a critical element inside the protein complex SNAREs vital to autophagic membrane fusion, preventing it from being transported onto autophagosome, and inhibiting the process of assembling with SNAP29, VAMP8 into SNARE compounds. Also, the interactions between STING-STX17 may be disturbed by energy deficiency, which attests that the autophagy induced by hunger controls the fusion efficiency of autophagic membrane by dint of STING-STX17’s interactions on the endoplasmic reticulum.
Besides, it is found through studies on STX17 in recent years that, such molecule not only mediates the fusion of autophagic membrane, but also, due to its phosphorylation by TBK1(TANK-binding kinase 1), involves in the forming process of mPAS, the initial structure of the autophagosome. Do the interactions of STING-STX17 bear on the formation of mPAS?
The researchers find that, the STX17 pS202 after being phosphorylated by TBK1 cannot bond with STING, but is more distributed in the initial autophagic structure marked by DFCP1(Double FYVE-containing protein 1); conversely, STX17 at S202 site in form of phosphorylation/deactivation exhibit better interactions with STING,and is moreover distributed in the complete autophagosomal structure marked by LC3+-LAMP2+(Lysosome-associated membrane glycoprotein 2). This part of experiment points out that STING may act on STX 17 protein not phosphoraylated by TBK1,thus bearing specifically on the autophagosome-lysosome fusion, other than act on the formation period of the autophagosome.
Coming next, the researchers investigate into whether the STING-STX17 interaction will be affected by cGAMP led level of activation of STING. The researchers find that STING-STX17 dissociation may occur on activation of STING molecules by cGAMP or emergence of ER-ERGIC transport of STING molecules,and that disease-related over-activation pattern exhibits strengthened interactions with STX17 and inhibition on autophagy. Moreover, the researchers find that STING C148 locus is the one vital to bonding with STX17, and the inactivating mutation of complementing STING C148 locus in knockout cells shows weak autophagic inhibition. This part demonstrates that activation of STING interferes with its bonding with STX 17, and also reveals the biochemistry mechanism of the STING-STX17 interactions.
In the end, the researchers find that, STING-STX17 action axis and STING molecule are independent each other in PAMPs-induced non-classical autophagy pathways, which is manifested as that, the STX 17 after HT-DNA-induced dissociation with STING is distributed in different autophagy-related structures, and that the knockout of STX17 does not affect the process of degradation and transport of STING molecules.
This research indicates that, with an environment of adequate nutrition and inactivated natural immunity, STING molecule can, via bonding with STX 17, a vital protein to autophagic membrane fusion, reside in endoplasmic reticulum, thus inhibiting STX 17 from involving in the autophagosome-lysosome fusion process, and down-regulating the autophagic flux. In the absence of STING, or activation of cGAMP and emergency of endoplasmic reticulum - ERGIC transport, STX17 is released by STING, which leads to up-regulation of autophagy level, and consequently strengthening body’s sugar metabolism and athletic ability in kinestate.
In summary, this research reveals a totally new non-immunological function of STING, that is, the spatial regulation effect on classical autophagy pathways caused by deficiency of energy, a clue to new relevance between congenital immunity and energy metabolism.
Professor Rong Yueguang with the Tongji Medical College of Huazhong University of Science and Technology, Doctor Zhang Shen, assistant researcher with Shanghai Jiao Tong University School of Medicine, Doctor Nilay Nandi with the Southwestern Medical Center of University of Texas, and Wu Zhe, doctoral student with the Tongji Medical College of Huazhong University of Science and Technology are the essay’s co-first authors. Researcher Zhong Qing with Shanghai Jiao Tong University School of Medicine, Professor Rong Yueguang with the Tongji Medical College of Huazhong University of Science and Technology, Professor Helmut Kramer with the Southwestern Medical Center of University of Texas, and Liu Xiaoxia, assistant researcher with Shanghai Jiao Tong University School of Medicine are the essay’s corresponding authors.

