

嗜酸性粒细胞是多功能粒细胞,在免疫调节、代谢平衡和组织修复等过程中发挥重要作用,然而其功能特化的调控机制仍不明确。
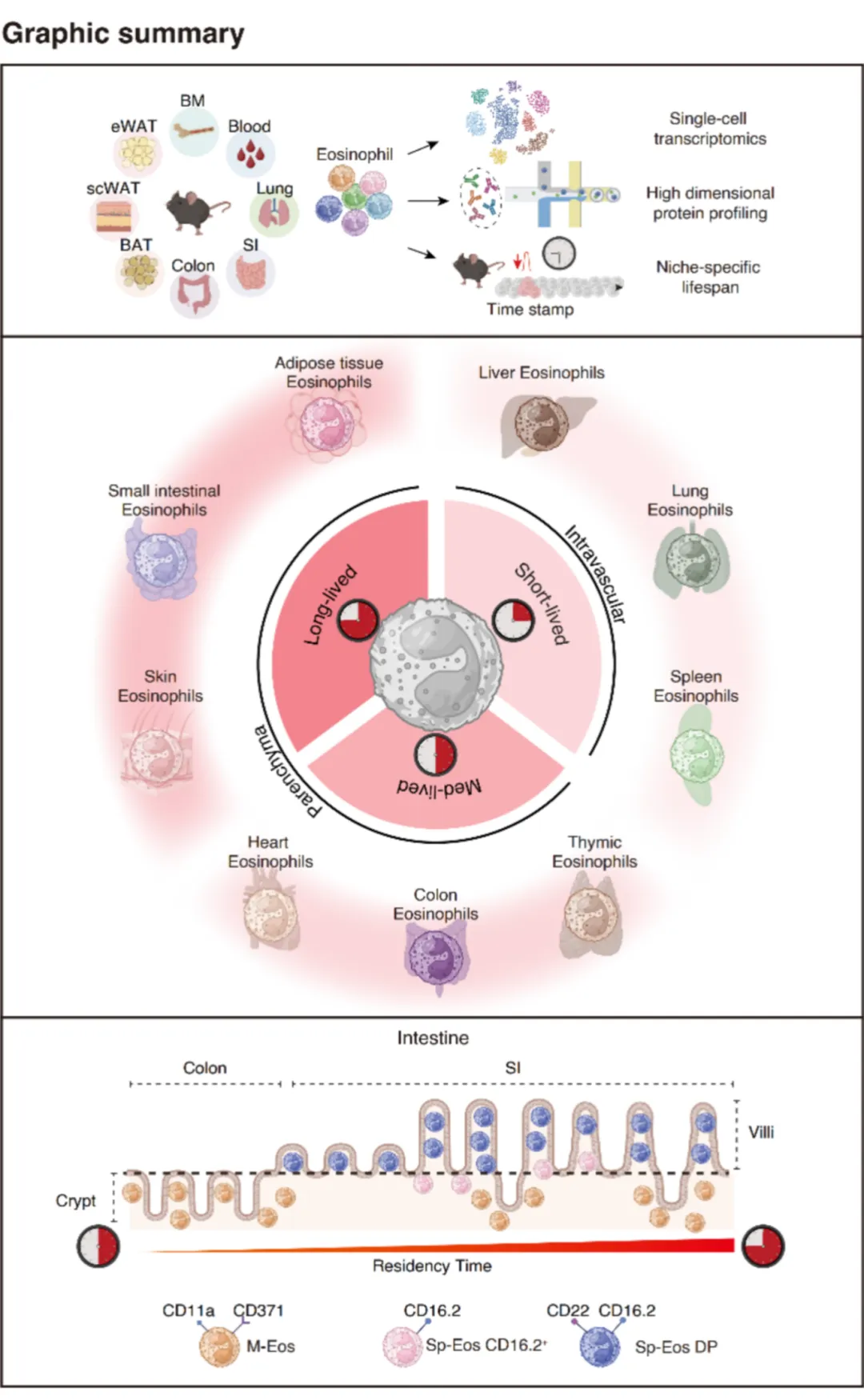
研究团队通过整合单细胞RNA测序、高维度表面蛋白组学和诱导性谱系示踪技术,系统描绘了嗜酸性粒细胞从骨髓前体、外周循环到组织定植的完整发育动态图谱。研究发现,组织驻留时间的延长促使嗜酸性粒细胞经历渐进式的功能特化,其异质性程度与细胞寿命呈显著正相关。短期驻留的嗜酸性粒细胞(短寿)维持相对均一的转录特征,而长期驻留的细胞(长寿)则表现出明显的层级化分子和表型特征。
为了系统阐明驻留时间依赖的细胞特化规律,本研究构建首个多组织嗜酸性粒细胞单细胞转录组-蛋白组联合图谱,整合分析了骨髓、血液及肺、小肠、结肠、皮肤和脂肪组织等多个器官的嗜酸性粒细胞,发现这些细胞呈现出显著的组织特异性分子印记和表面蛋白特征,首次实现了不同组织来源嗜酸性粒细胞亚群的前瞻性分选。此外,研究团队建立了一套经过严格验证的生物标志物组合,鉴定了一系列与分化轨迹相关的标记物——包括CD371、CD11a、CD22和CD16.2——这些标记物能够有效区分嗜酸性粒细胞不同发育阶段的细胞亚群—前体、循环型和特化的组织驻留型。将嗜酸性粒细胞研究从长期缺乏有效工具的领域,推进至具备单细胞分辨率的研究体系。
同时,该研究首次系统阐明了嗜酸性粒细胞在不同组织中的动态驻留特征与显著差异,具体表现在:肺嗜酸性粒细胞寿命短,主要位于血管内,且转录上均一;结肠嗜酸性粒细胞寿命中等,呈现有限的特化;小肠嗜酸性粒细胞长寿,主要位于肠实质层内,并分化为多个转录和表型上截然不同的亚群。通过谱系示踪技术,研究者建立了嗜酸性粒细胞的寿命与特化状态的联系,证实组织微环境的持续作用时间是驱动嗜酸性粒细胞多样化的关键决定因素。
进一步研究发现,驻留时长驱动的特化过程受到空间组织的精细化调控。在小肠内,长寿嗜酸性粒细胞优先定位于肠绒毛,而短期驻留的细胞则局限于隐窝。沿着肠道轴向,肠绒毛的消失与结肠中嗜酸性粒细胞寿命的缩短及异质性的降低相一致。这些结果表明,嗜酸性粒细胞的命运决定受到"微解剖定位-驻留时长"协同调控机制的精确控制。该发现首次在嗜酸性粒细胞中证实了空间微环境对细胞功能特化的调控作用,将基于微环境的免疫调控理论成功拓展至嗜酸性粒细胞研究领域。
综上,本研究首次将组织驻留时长确立为嗜酸性粒细胞功能分化的核心调控维度,这一发现与当前巨噬细胞和中性粒细胞研究中的前沿概念形成重要呼应。通过系统性构建首个跨组织单细胞多组学整合图谱(涵盖转录组和蛋白组),揭示了驻留时长是驱动嗜酸性粒细胞特化的主导因素。这一突破性成果为深入解析嗜酸性粒细胞在多重生理病理过程中的作用机制——包括但不限于粘膜屏障免疫、系统代谢调控、炎症反应和组织修复——提供了不可或缺的基础研究工具。为确保该基础研究成果的资源共享和广泛应用,本研究已将数据上传至 CNGBdb的可交互式公共数据平台。
全文链接:https://rdcu.be/eYjeb
上海市免疫学研究所Svetoslav Chakarov课题组博士研究生胡雅楠为文章的第一作者, 上海市免疫学研究所Svetoslav Chakarov研究员与中国医学科学院苏州系统医学研究所李子逸研究员为该论文的共同通讯作者。该研究得到了上海市免疫学研究所流式、成像平台和上海交通大学医学院基础医学院公共技术平台和实验动物中心,苏州系统医学研究所高性能计算平台的技术支持,以及科技部重点研发计划、国家自然科学基金等项目资助。

Svetoslav Chakarov,上海交通大学医学院/上海市免疫学研究所课题组长、博士生导师,入选国家海外高层次人才计划。其研究团队致力于解析髓系细胞的异质性、分化途径及其驻留的亚组织微环境如何调控组织特异性代谢功能的机制。以第一作者或通讯作者身份在Science(2019年、2025年)、Immunity、J.Exp.Med.、PNAS等国际权威期刊发表原创性研究成果。主持国家自然科学基金外国学者研究基金项目资助,作为项目骨干参与科技部重点研发计划,并获上海市外国专家项目资金支持。
Time of Tissue Residency as a Fundamental Driver of Eosinophil Specialization
A collaborative team led by Dr. Svetoslav Chakarov from the Shanghai Institute of Immunology, together with national and international partners, has published a landmark Resource paper in Nature Immunology entitled “Temporal and spatial atlas of eosinophil specialization across tissues.”The study establishes time of tissue residency as a core principle governing eosinophil maturation, diversity, and function, fundamentally reshaping how eosinophil biology is understood across organs. To uncover this principle, the authors generated the first integrated single-cell transcriptomic and proteomic atlas of eosinophils across multiple tissues, providing an unprecedented view of eosinophil life history in vivo.

Eosinophils are multifunctional granulocytes implicated in immunity, metabolism, and tissue repair, yet the rules governing their specialization have remained unclear. This study demonstrates that how long eosinophils persist within a tissue niche is a dominant determinant of their functional diversification, acting in concert with local environmental cues. By combining single-cell RNA sequencing, high-dimensional surface proteomics, and inducible fate mapping, the authors show that eosinophils follow a conserved differentiation continuum from bone marrow to blood and into tissues, where extended residency enables progressive specialization. Short-lived eosinophils remain transcriptionally uniform, whereas long-lived eosinophils acquire layered molecular and phenotypic identities.
To systematically define residency-linked specialization, the authors constructed the first multi-organ single-cell and proteomic atlas of eosinophils, profiling cells from bone marrow, blood, lung, small intestine, colon, skin, and multiple adipose depots. This atlas reveals that eosinophils adopt tissue-imprinted transcriptional and surface protein signatures, but crucially, the degree of heterogeneity scales with lifespan. The work also delivers validated marker panels that enable prospective isolation of eosinophil subsets across tissues. Additionally, the research team established a rigorously validated biomarker panel and identified a series of markers associated with differentiation trajectories—including CD371, CD11a, CD22, and CD16.2—that distinguish progenitor, circulating, and specialized tissue-resident eosinophils. This work advances eosinophils from an underrepresented cell type into a tractable system for single-cell analysis.
The study uncovers striking contrasts between tissues with different residency dynamics:
Lung eosinophils are short-lived, largely intravascular, and transcriptionally uniform.
Colon eosinophils exhibit intermediate residency with limited diversification.
Small intestinal eosinophils are long-lived, parenchymal, and diversify into multiple transcriptionally and phenotypically distinct subsets.
Using fate mapping, the authors directly link eosinophil lifespan to the emergence of specialized states, demonstrating that extended tissue retention is required for eosinophil diversification.
Residency-driven specialization is further sharpened by spatial organization. Within the small intestine, long-lived eosinophils preferentially localize to villi, while short-lived populations remain confined to crypts. Along the intestinal axis, the loss of villi coincides with reduced eosinophil lifespan and diminished heterogeneity in the colon. These findings show that microanatomical localization and residency time act together to instruct eosinophil fate, extending niche-based principles of immune regulation to granulocytes.
This work positions time of tissue residency as a central axis of eosinophil specialization, analogous to emerging concepts in macrophage and neutrophil biology. By delivering the first cross-tissue single-cell and proteomic eosinophil atlas and uncovering residency time as a governing principle, the study provides a foundational resource for investigating eosinophil roles in barrier immunity, metabolism, inflammation, and tissue repair. An interactive public portal accompanies the study, enabling broad community access to this resource.

Svetoslav Chakarov, Principal Investigator and Doctoral Supervisor at the Shanghai Jiao Tong University School of Medicine / Shanghai Institute of Immunology, is a recipient of the National High-Level Talent Program. His research group is dedicated to understand the heterogeneity of myeloid cells, their differentiation pathways and how their sub-tissular niche of residence dictates their tissue specific metabolic functions. He has published original studies as first or corresponding author in leading international journals including Science(2019, 2025)Immunity, J. Exp. Med. and PNAS. He currently heads projects funded by the National Natural Science Foundation of China, has been awarded , Research Fund for International Scientists, participates as a key member in Ministry of Science and Technology National Key R&D Program of China, and has received financial support under the Shanghai Foreign Expert Program.
 沪公网安备 31009102000053号 沪ICP备18007527号-1 邮箱:sii@shsmu.edu.cn
沪公网安备 31009102000053号 沪ICP备18007527号-1 邮箱:sii@shsmu.edu.cn