Dr. Qiming Liang's team from Shanghai Institute of Immunology, Shanghai Jiao Tong University School of Medicine together with Dr. Zhen Zhao's Lab from Zilkha Neurogenesis Institute, University of Southern California Keck School of Medicine, published their research on Cell Stem Cell on August 6, 2020, entitled “The Zika Virus Capsid Disrupts Corticogenesis by Suppressing Dicer Activity and miRNA Biogenesis”. The study showed that ZIKV Capsid protein interacts with Dicer and inhibits its miRNA processing activity, especially blocks the production of neurodevelopment-related miRNAs, suppressing neurogenesis and subsequently leading to microcephaly.

Zika virus (ZIKV), a mosquito-borne flavivirus, was firstly isolated from a febrile rhesus macaque in the Zika forest of Uganda in 1947. However, until the outbreak on Yap Island in 2007, only 14 cases of human infection had been reported and these patients just appeared some mild symptoms, such as skin rashes, conjunctivitis, muscle or joint pain. In 2015, this virus re-outbroke in South American regions, especially in Brazil, and it showed a tendency of spreading to North America and even worldwide in one year. Unfortunately, the recent outbreak of this virus was associated with dramatic clinical increase of severe fetal malformation, including spontaneous abortion, stillbirth, microcephaly, hydrocephaly, and so on. Meanwhile, ZIKV was detected in the amniotic fluids of pregnant women as well as in the brain tissues of microcephalic fetuses. Therefore, the World Health Organization (WHO) declared that ZIKV became a global health emergency in February 2016, which had greatly accelerated the global research on ZIKV. In a short period of time, a variety of ZIKV infection models in mice and non-human primates had been established, and they had been applied to the diagnosis and drug screening. So far, however, the pathogenesis of ZIKV causing microcephaly is still unclear, and the related molecular mechanisms, especially the mechanisms of nervous system infection, are very limited, which seriously hinders the drug development for ZIKV.
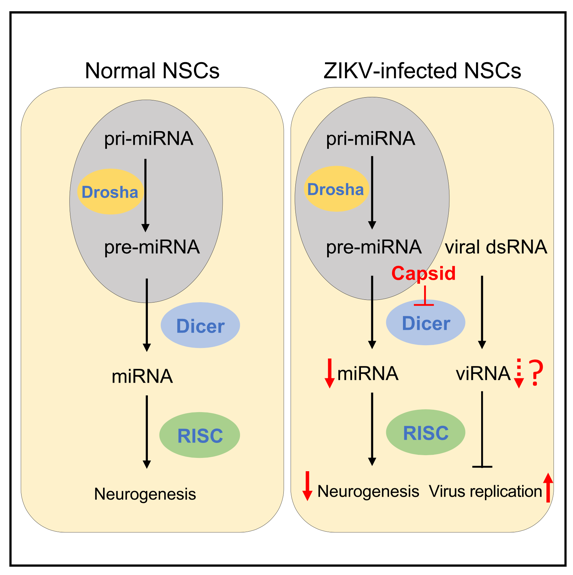
RNA interference (RNAi) is an important biological process in many eukaryotes during which small RNA molecules neutralized targeted mRNA molecules to inhibit the translation or gene expression. Dicer, a fundamental miRNA biogenesis enzyme, initiates the RNAi pathway by cleaving dsRNAs or stem-loop structure of precursor miRNAs (pre-miRNAs) into short dsRNAs, and one strand of dsRNAs is directly loaded into the RNA-induced silencing complex (RISC). The mature miRNA perfectly or partially pairs with complementary sites on target mRNAs, leading to the inhibition of mRNA stability or translation (Figure 1). miRNAs are involved in almost every cellular process and are essential for cell differentiation, development and homeostasis. Deregulation of miRNA is associated with numerous diseases in human. For example, Dicer deficiency leads to microcephaly in animal models; and several miRNAs, e.g. let-7a, miR-9, miR-17, and miR-19a, play crucial roles during fetal brain development. Moreover, dysregulation of host miRNA after ZIKV infection has been recently documented in NSCs, astrocyte-like SVG-A cells and mosquitoes. However, whether there is a direct link between Dicer-dependent miRNA system and ZIKV induced microcephaly is still unknown.
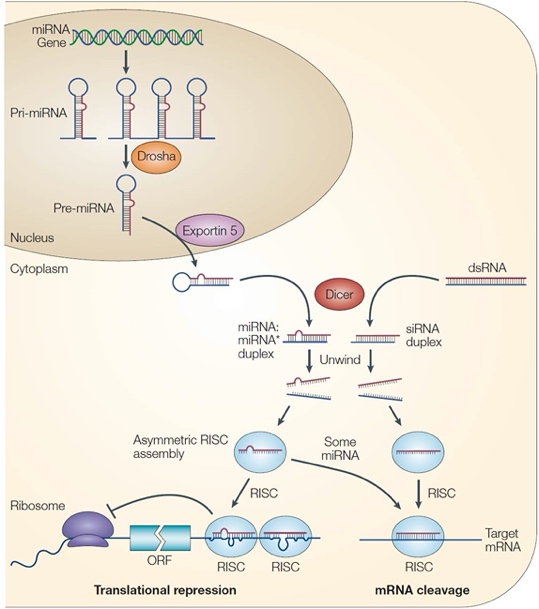
Figure 1: Process of miRNA biogenesis
By analyzing the ZIKV-host interactome in neural stem cells (NSCs) (Figure 2), Dicer was identified as the leading target of capsid. ZIKV Capsid protein specifically interacts with the endonuclease Dicer, which do not appear in other flaviviruses Capsid protein, such as Dengue fever virus (DENV), Japanese encephalitis virus (JEV), West Nile virus (WNV), etc. This specific interaction can inhibit Dicer's miRNA processing activity, and hinder the synthesis of mature miRNAs, especially a serious of miRNAs related to neurogenesis (e.g.let-7a, miR-9, miR-17).
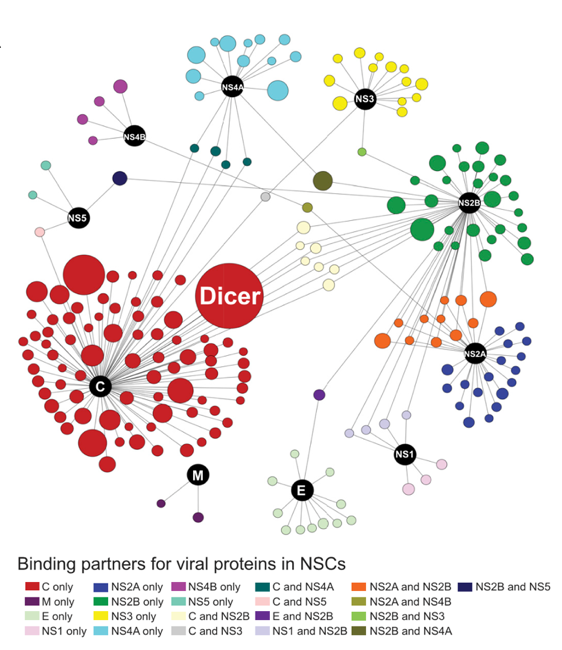
Figure 2: ZIKV-host interactome in neural stem cells
The researchers compared the amino acid sequence of the Capsid protein of ZIKV with other typical flaviviruses and found that Capsid contains a single mutation at residue 41(H41R) lost its interaction with Dicer and no longer inhibit Dicer cleavage activity. Then, they generated ZIKV-WT and ZIKV with H41R mutation in Capsid through viral reverse genetics and infected the two into the embryonic brain at 13.5 days (E13.5) respectively through in utero microinjection (Figure 3). By measuring the size of the fetal brain and the thickness of each brain layer at E18.5, they found that the ZIKV-WT caused smaller fetal mouse brain and thinner cerebral cortex compared to the mutant virus, indicating that the inhibition of Dicer by Capsid protein indeed causes neurodevelopmental defects. In addition, in order to verify whether the ZIKV Capsid protein itself is sufficient to cause neurodevelopmental defects in vivo, they used the AAV gene delivery system to express the Capsid protein and its H41R mutant in the embryonic brains and found that the Capsid protein itself is sufficient to cause neurodevelopmental defects.
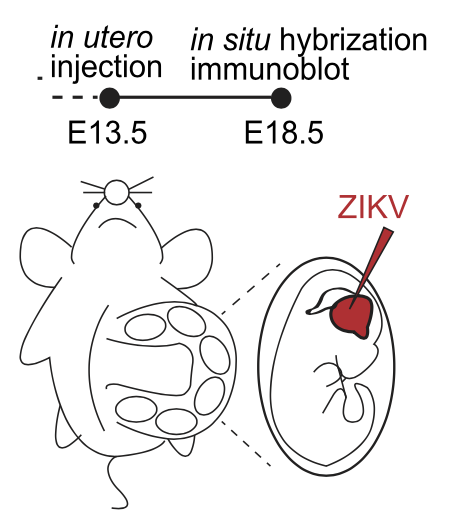
Figure 3: Diagram of uterine microinjection
Conclusion: The study showed that ZIKV Capsid protein specifically interacts with Dicer to inhibit its miRNA processing activity, especially blocks the production of neurodevelopment-related miRNAs, suppressing neurogenesis and leading to microcephaly.
By Shupeng Dong