On May 20, 2020, the Wang Honglin group of Shanghai Institute of Immunology, Shanghai Jiao Tong University School of Medicine, published a research article entitled "A Micropeptide Encoded by LncRNA MIR155HG Suppresses Autoimmune Inflammation via Modulating Antigen Presentation" in Science Advances, reporting that a previously annotated non-coding region of the human genome encodes a polypeptide named miPEP155. miPEP155 can regulate the antigen-presenting capacity of dendritic cells in the context of inflammation. miPEP155 showed prominent therapeutic effects on two autoinflammatory disease models, namely psoriasis-like mouse model and experimental autoimmune encephalomyelitis (EAE), have good therapeutic effects, highlighting the potential of miPEP155 to be a drug candidate of autoimmune diseases.
miRNA precursors are generally considered to be non-coding. Recent studies have shown that the small open reading frames within miRNA precursors and other lncRNAs might harbor the ability to encode polypeptides. Several biologically active peptides derived from lncRNAs, such as immunoactive peptides and neuroactive peptides, have been discovered. There are 1881 miRNA precursors and more than 20,000 lncRNAs in the human body, which brings out the possibility that the polypeptides encoded by "non-coding regions" even exceed all known types of proteins, greatly expanding our understanding of the polypeptides and their functions in vivo. In 2015, Dominique et al. reported in Nature that miR-165a and miR-171b precursors encode polypeptides that regulate Arabidopsis root development. However, whether the same phenomenon exists in mammals still needs further study.
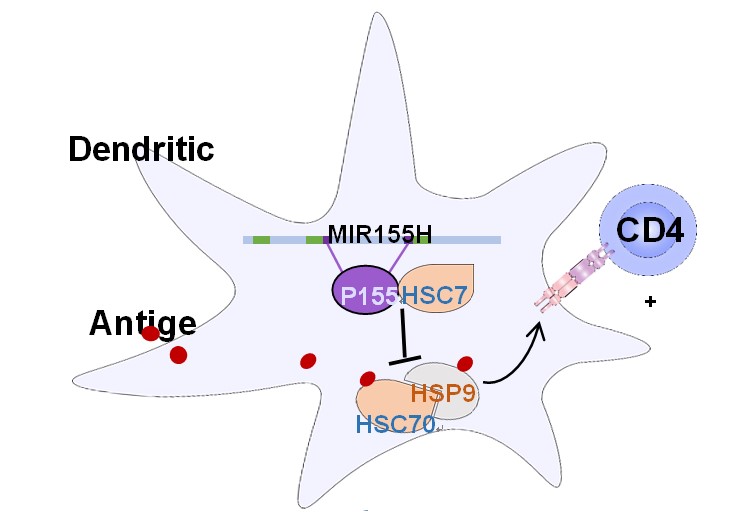
Figure 1. miPEP155 interacts with HCS70 to regulate antigen presentation and treat autoimmune diseases.
Using bioinformatics analysis and exploring Ribo-seq datasets, the authors discovered the potential for encoding peptides in the "non-coding" gene miR-155 host gene (MIR155HG) in human dendritic cells (DCs). Further through CRISPR-Cas9-facilitated genetic editing, customized antibody detection and other means, the authors confirmed for the first time that a human "non-coding" microRNA precursor can encode a polypeptide, and the polypeptide encoded by MIR155HG was named miPEP155. The authors used biotin-labeled miPEP155 to discover its interacting protein HSC70 in DCs, which was verified in the setting of endogenous miPEP155. HSC70 is a molecular chaperone and heat shock cognate, harboring two main domains, ATPase domain and substrate-binding domain (SBD). HSC70 is shown to be central in antigen transportation and presentation processes. The authors found that miPEP155 can specifically bind to the ATPase domain of HCS70, greatly reducing antigen transportation and MHC class II membrane expression in DCs, thereby regulating antigen presentation in auto-inflammation conditions (Figure 1).
Psoriasis is a common chronic cutaneous auto-inflammatory disorder characterized by epidermal hyperplasia and massive dermal immune infiltration. Psoriasis is rarely fatal, but this incurable disease severely impacts patients’ quality of life. In a mouse model of psoriasis induced by imiquimod, miPEP155 treatment significantly reduced the thickness of the skin and the infiltration of inflammatory cells in the dermis. DCs are the innate immune cells with the strongest antigen presenting ability and skin DCs play a vital role in the pathogenesis of psoriasis. miPEP155 alleviated psoriasis-like skin inflammation by inhibiting antigen presentation executed by DCs, and the same mechanism was employed by miPEP155 to treat EAE, which is a classical mouse model mimicking human autoimmune disease–multiple sclerosis.
Peptides have the advantages of minimal deposit in the human body, limited side effects, low effective dosage, fast absorption, and low production cost. The global peptide drug market has exceeded 20 billion US dollars and maintains a 10% annual growth rate. Peptide drug candidates are involved in the development of vaccines, anti-tumor drugs, cardiovascular and cerebrovascular drugs, antiviral drugs, as well as antibacterial drugs and diagnostic kits. Nearly 60 peptide drugs have been approved worldwide. Wang Honglin group demonstrated miPEP155 to be a drug candidate for autoimmune diseases with the drug target elucidated and curative effects confirmed.
Wang Honglin, Distinguished Professor and PI of Shanghai Institute of Immunology, Shanghai Jiao Tong University School of Medicine, winner of the National Outstanding Youth Fund, has long been engaged in psoriasis research, and has achieved a series of original findings in the field of psoriasis etiology and new therapeutic target identification. The current research was independently carried out by Wang Honglin group, and the findings have strong translational potentials. Wang Honglin group also has achievements in the field of new drug discovery and development, and has the experience and strength to successfully obtain the national class I new drug clinical approval. Dr. Wang Honglin is the corresponding author of the article. Postdoctoral fellows Niu Liman, Lou Fangzhou, and assistant researcher Sun Yang are co-first authors of the paper. The research was supported by grants from the National Natural Science Foundation of China and Shanghai Collaborative Innovation Center for Translational Medicine, Innovative Research Team of High-Level Local Universities in Shanghai. This work was also supported by the flow core, imaging core and sequencing core of Shanghai Institute of Immunology.