Keywords
Pancreatic cancer, cancer stem cells, circulating tumor cells, precision medicine
Research Activities
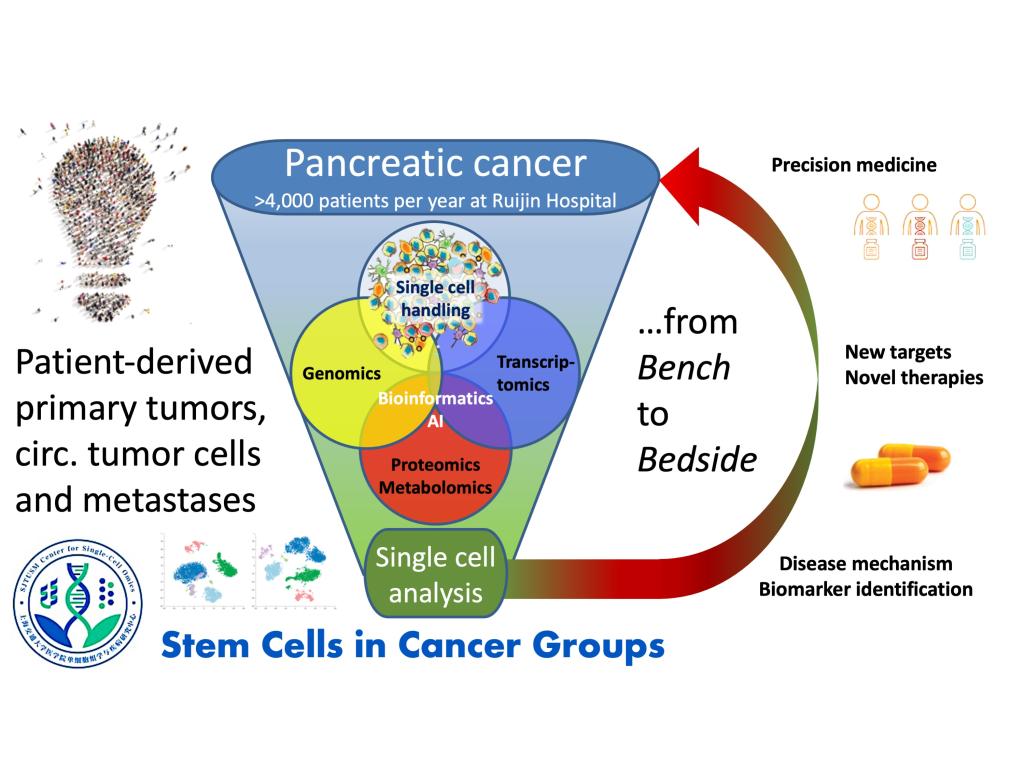
We are aiming to improve the outcome of pancreatic cancer, a disease with clear unmet medical need. We are using cutting-edge technologies including single-cell omics to dissect the heterogeneity of pancreatic tumors and develop more effective therapies for our cancer patients.
It has now become evident that cancer heterogeneity is not only related to the existence of different sub clones within the tumor, but heterogeneity is further enhanced by so-called cancer stem cells at the subclonal level. These cells are responsible for intraclonal heterogeneity, promote metastasis and are inherently resistant to conventional therapies.
We pursue large-scale and high-resolution interrogations of (metastatic) pancreatic cancer cells and their tumor microenvironment using fresh patient material, single-cell omics, and complementary approaches for functional taxonomy. We aim to define the landscape and epigenetic trajectory of stemness and therapeutic resistance via non-genetic evolution. This provides the basis for developing novel, more effective treatment strategies (e.g. Cell Stem Cell 2007, 2011, 2015, Cell Metab 2015, 2021, Cancer Cell 2008, 2014, 2016, Nat Methods 2014, Nat Comm 2018, 2019, 2020, 2021).
The three overarching high-level objectives of our research program are:
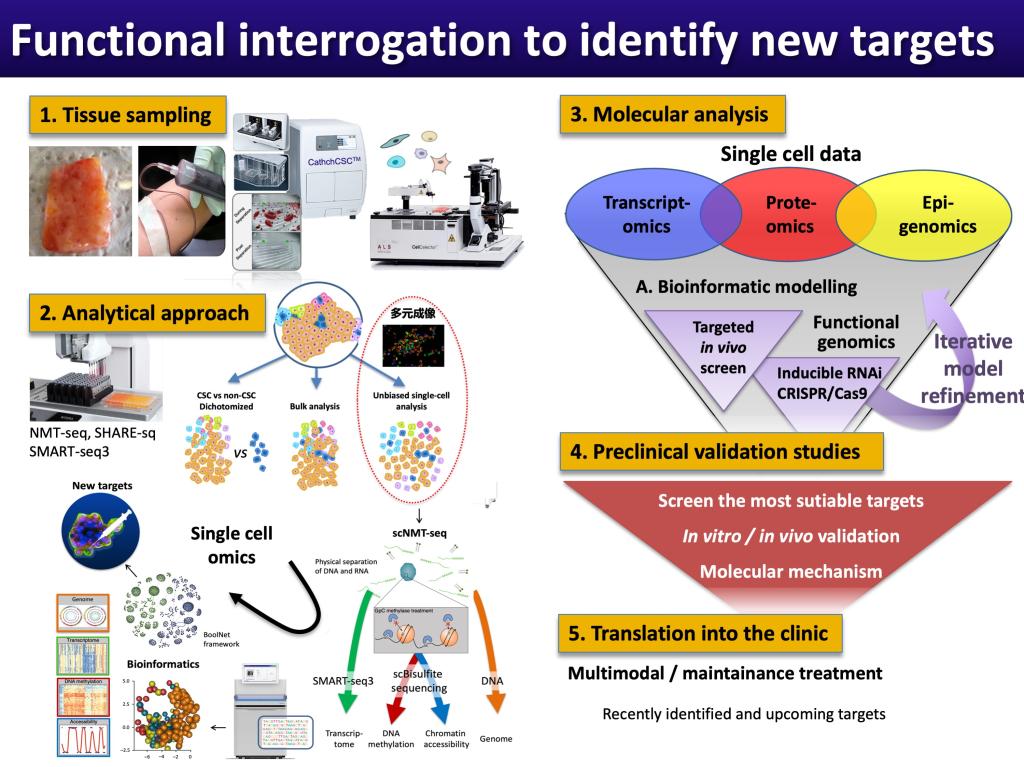
1. Identify and validate novel and common targets that are vital for cancer stem cell functions.
2. Develop multimodal therapeutic strategies to jointly eliminate cancer stem cells, differentiated cancer cells and the protective stroma.
3. Test these novel treatment strategies using precision medicine approaches.
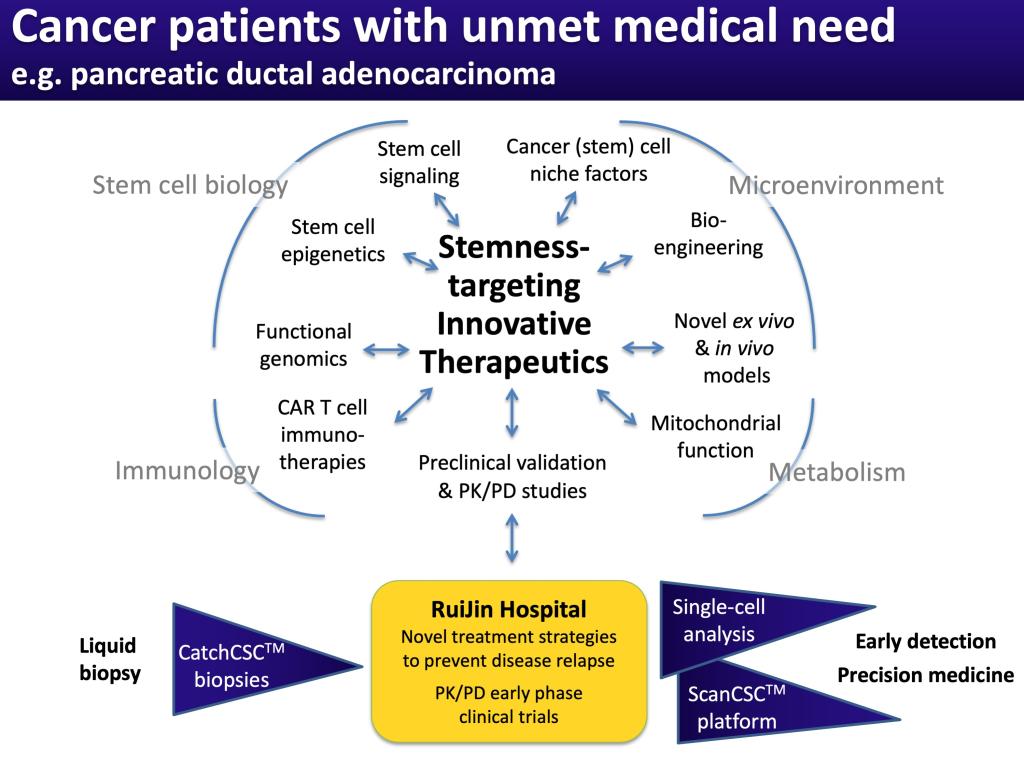
The underlying hypothesis for our research program is that comprehensive functional interrogation of cancer stem cells results in the identification of novel targets with broad applicability. Inhibition of newly identified pathways will prevent disease progression and/or relapse during treatment.
Significant funds provided by the SJTU School of Medicine and the Shanghai Government have been invested to bring Prof Heeschen and his team to the Center for Single-Cell Omics, providing a state-of-the-art research environment. We operate intestines suites of equipment, including cutting-edge facilities for the isolation, handling and propagation of rare (circulating) cancer stem cells, and their subsequent comprehensive molecular and phenotypic analysis.
Available positions
We are continuously looking for skilled personal at all levels:
§ Assistant / Associate Professors
§ Postdocs
§ PhD students
§ Master students
Available projects are focusing on:
§ Dissect the heterogeneity of circulating cancer (stem) cells at single-cell level;
§ Bioinformatic analysis of large-scale single-cell omics data to create in silico models
§ Study genetic and epigenetic evolution / trajectory of metastasis and drug resistance;
§ Identifying novel therapeutic targets using single-cell functional genomics;
o Epigenetic regulation of stemness / metastasis
o Metabolism in cancer stem cell
o Immunology of cancer stem cells
§ Design and test novel immunotherapies (e.g. tuneable CAR T-cells);
§ Disrupting the tumor microenvironment to enhance treatment response;
§ Develop novel 3D multicellular models for precision medicine approaches.
For inquiries, please contact me at christopher.heeschen@icloud.com
Our Publications can be found on PubMed
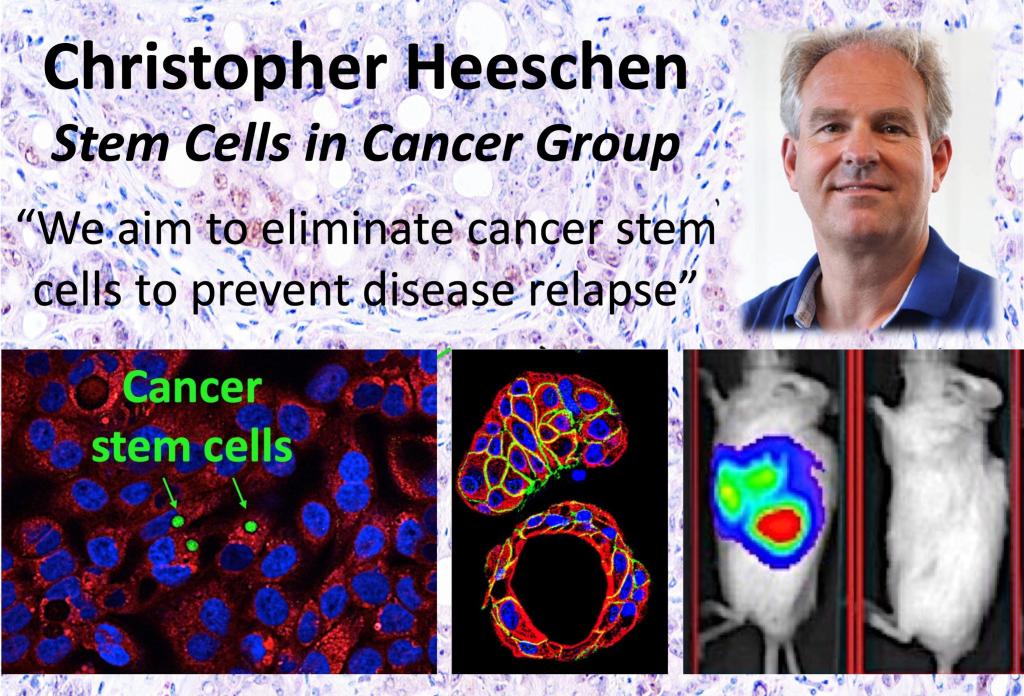
Prof Heeschen’s Biography
Prof Heeschen was born in Germany and obtained his MD in 1997 at the Free University of Berlin (Germany). His clinical training in Internal Medicine ran from 1996 – 2004 at the University of Hamburg and Frankfurt, including a DFG Research Fellowship at Stanford University (USA) and earning his PhD in 2003.
Prof Heeschen became an independent investigator in 2004 as Professor for Experimental Oncology and Head of the Department of Experimental Medicine at Ludwig-Maximilian-University in Munich (Germany). In 2008, he moved to the Spanish National Cancer Research Centre (CNIO) as Founding Director of the Clinical Research Programme. In 2013, he became the Lead of the new Centre for Stem Cells in Cancer & Ageing at Barts Cancer Institute, London, UK. In October 2019, Prof Heeschen was recruited to Shanghai Jiao Tong University to establish his translational research program at the Center for Single-Cell Omics.
Prof Heeschen has published more than 170 articles in prestigious scientific journals such as the New England Journal of Medicine, The Lancet, Nature Medicine, Cancer Cell, Cell Stem Cell, among others. His work has been cited more than 33,000 times and currently his H-index is 74. Prof Heeschen has made seminal contributions to the field of stem and vascular cell biology that have advanced our understanding of the basic processes of stem cell function and trafficking. His research has been recognized through numerous international awards and has earned him two successive ERC Advanced Investigator Grants for pancreatic cancer research.
Background
Pancreatic cancer still has the worst outcome of any cancer in China and the world. At the same time, the incidence of pancreatic cancer keeps increasing, with currently ~448,000 new cases in 2020. This number is predicted to further increase in the coming years. Due to its extreme lethality with no effective treatments available at present, pancreatic cancer is currently the 3rd most frequent cause of cancer-related deaths, but may even become the 2nd most frequent cause by 2030. Pancreatic tumors are heterogeneous, not only because of diverse subclones that arise during tumor evolution, but also because they are driven by functional heterogeneity within each subclone. So-called cancer stem cells (CSC) are responsible for intraclonal functional heterogeneity.
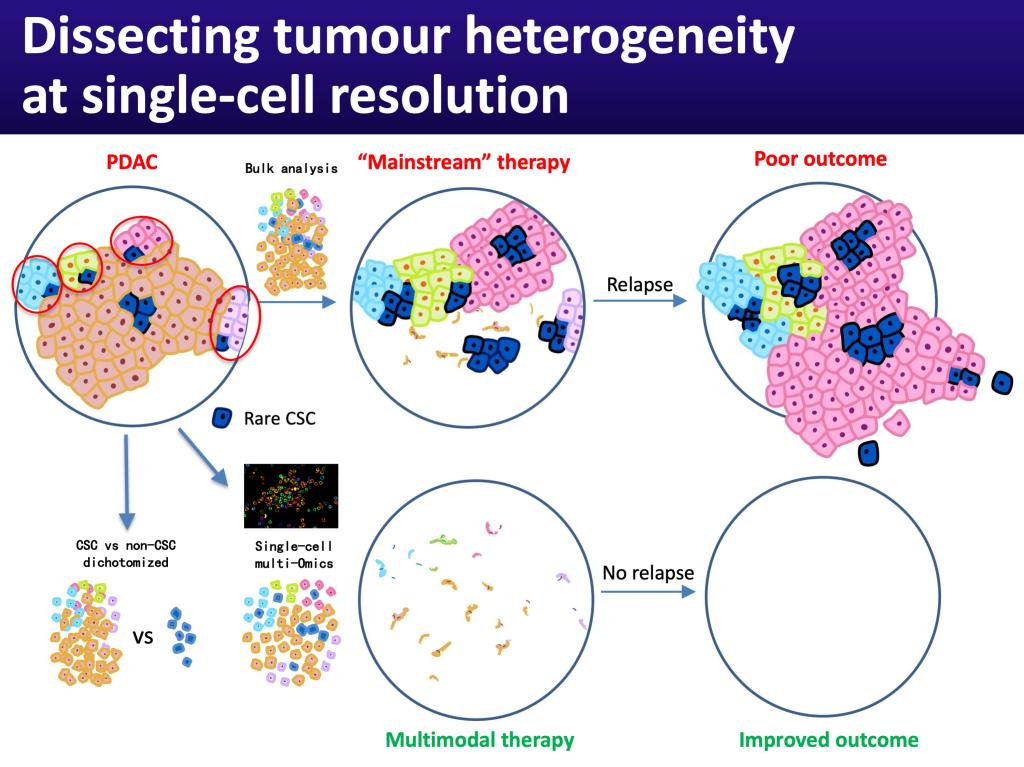
Traditionally, tumors are analyzed as a whole, which can result in overlooking the contained smaller clones and, most importantly, rare cancer stem cells. This approach has led to the development of therapies that mostly address dominating clones and cell populations. However, the remaining cancer stem cells can then quickly re-establish the tumor resulting in disease relapse.
With the emergence of the cancer stem cell concept, investigators including ourselves started dichotomizing tumors into cancer stem cells versus non-cancer stem cells. While this is certainly better than bulk analysis of tumors, it still misses considerable information as distinct subsets of cancer stem cells exist in each subclone. Now with the availability of new technologies we are able to analyze tumors using cutting-edge single-cell omics platforms. This allows us to detect and characterize rare clones, including the contained cancer stem cells. Notably, we are phenotypically characterizing the cells prior to single cell analysis using multiplex imaging. This allows us to later physically dissect cancer heterogeneity using these surface markers.
The obtained vast information provides the basis for the development of multimodal therapies that are capable of eliminating all cells, including highly aggressive cancer stem cells. As such, our studies are directly relevant to improving therapy in pancreatic cancer. We will provide vital and novel information on how to efficiently target cancer stem cells, and, in collaboration with Prof Bai-Yong SHEN at Ruijin Hospital, translate our findings into the clinic, by providing thorough scientific evidence for the design of biomarker-guided Phase I-II precision medicine trials. This will directly address and improve the still devastating prognosis of pancreatic cancer patients.