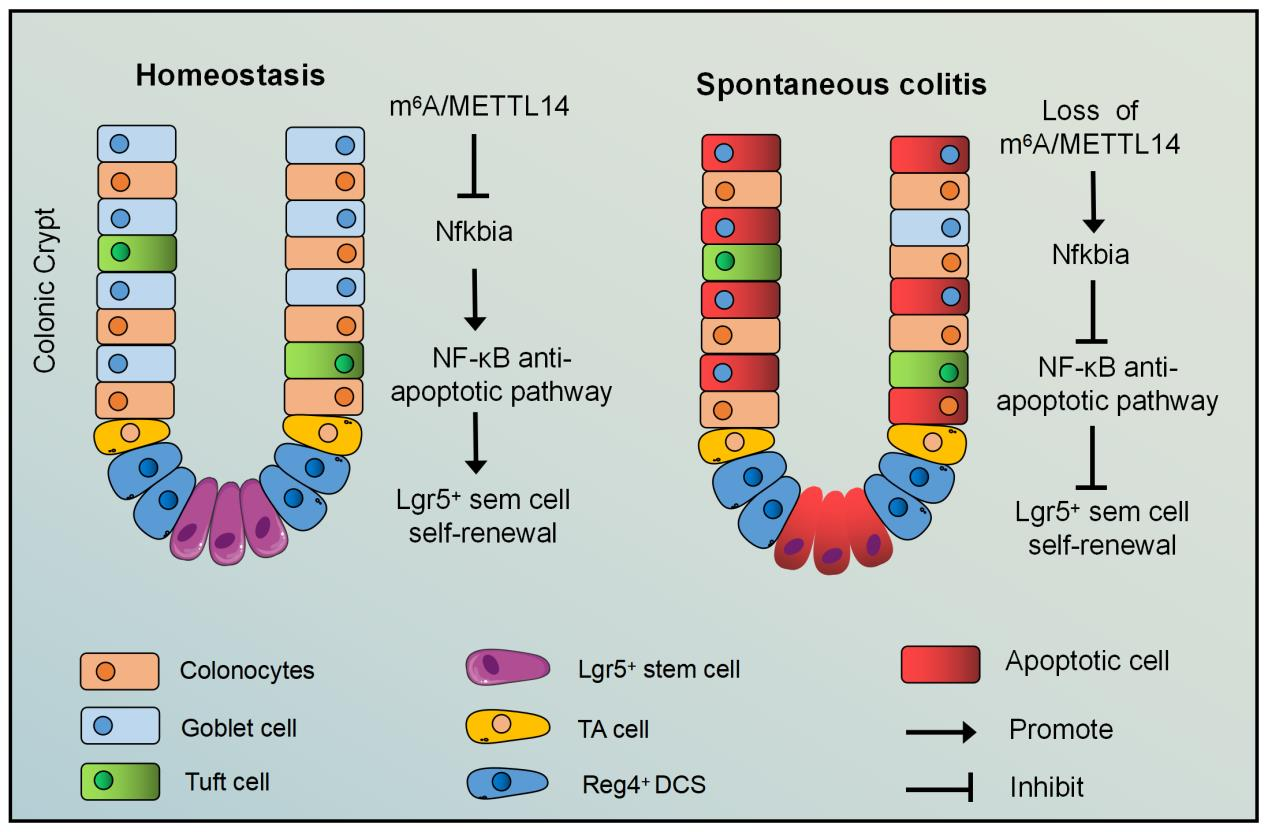
The article m6A mRNA modification maintains colonic epithelial cell homeostasis via NF-κB-mediated anti-apoptotic pathway was published in Science Advances by PI Huabing Li Lab of Shanghai Jiaotong University School of Medicine/Shanghai Institute of Immunology on 25 March 2022, demonstrated that m6A methyltransferase METTL14 played a crucial role in colonic epithelial development, apoptosis and spontaneous colitis, promoting colonic epithelial development, maintaining intestinal epithelial homeostasis and inhibiting colitis.
Abstract
Colonic mucosal barrier dysfunction is one of the major causes of inflammatory bowel disease (IBD). However, the mechanisms underlying mucosal barrier dysfunction are poorly understood. N6-methyladenosine (m6A) mRNA modification is an important modulator of epitranscriptional regulation of gene expression, participating in multiple physiological and pathological processes. However, the function of m6A modification in colonic epithelial cells and stem cells is unknown. Here, we show that m6A modification is essential for maintaining the homeostatic self-renewal in colonic stem cells. Specific deletion of the methyltransferase 14 (Mettl14) gene in mouse colon resulted in colonic stem cell apoptosis, causing mucosal barrier dysfunction and severe colitis. Mechanistically, we revealed that Mettl14 restricted colonic epithelial cell death by regulating the stability of Nfkbia mRNA and modulating the NF-κB pathway. Our results identified a previously unidentified role for m6A modification in colonic epithelial cells and stem cells, suggesting that m6A modification may be a potential therapeutic target for IBD.
Link: https://www.science.org/doi/10.1126/sciadv.abl5723
About Lab
Laboratory of RNA Epigenetics &Immunity
There are more than 100 types of different chemical RNA modification, among which m6A, m1A, m5C, pseudoUridine and APA are the major ones. HBL lab has made a series of pioneering discoveries in the m6A studies, won international reputations in the field, and laid solid experimental foundation. m6A research has been the frontier of the epigenetics field, but it is under explored for other types of RNA modifications, especially the cross-talks among those RNA modifications in response to environmental stimulation are unknown. Immune cells, especially T cells and macrophages in various inflammatory disease and tumor, provide an ideal perspective to explore this frontier. Combine mouse reverse genetics and state-of-art omics techniques, the research focuses on the following areas:
1) Function of m1A modification in T cells and macrophages;
2) Function of pseudouridine modification in T cells and macrophages;
3) Function of APA modification in T cells and macrophages;
4) Develop inhibitors to those RNA modification and apply to tumor immunotherapy;
5) Cross-talks of those RNA modifications.