Shanghai Jiao Tong University School of Public Health, in collaboration with the China National Center for Food Safety Risk Assessment, systematically assessed the dietary exposure sources and health risks of per- and polyfluoroalkyl substances (PFASs) for infants and school-aged children, based on the first long-term follow-up birth cohort established in a PFAS-highly polluted area in China. The findings were recently published in the Environmental Health Perspectives and Environmental Chemistry and Ecotoxicology, both of which are top-tier journals in the field of environment and health. By measuring PFAS levels in breast milk and local foods, the study identified the primary dietary exposure sources for local residents, providing critical scientific evidence for protecting vulnerable populations and formulating intervention strategies.
PFASs are a class of synthetic organic fluorides widely used in various consumer and industrial products. Due to their persistence in the environment and their endocrine-disrupting effects, PFASs have been classified as globally prioritized environmental endocrine-disrupting chemicals. China’s Ministry of Ecology and Environment has also listed them as the top priority in the List of Key Controlled Emerging Pollutants (2023).
The research team led by Prof. Ying Tian and Prof. Yu Gao from the School of Public Health at Shanghai Jiao Tong University established the Laizhou Wan Birth Cohort (LWBC) and conducted a 10-year prospective follow-up study on the health effects of prenatal PFAS exposure on offspring. Their findings revealed that PFAS levels in pregnant women and fetuses were significantly tens of times higher than those reported in similar domestic and international studies. Moreover, these elevated levels were associated with adverse health outcomes in offspring. Dietary exposure assessment indicated that food was the primary source of PFAS exposure for the local population, though the specific high-risk food categories and their contamination levels remained unclear. To address this gap, the research team further measured PFAS levels in breast milk and local foods to identify dietary exposure sources and assess health risks for infants and children.
For infants, breast milk is the main route of PFAS exposure. While existing research has primarily focused on the health risks of prenatal PFAS exposure, less attention has been paid to postnatal exposure via breast milk and its health impacts. Additionally, although school-aged children share similar dietary patterns with adults, their higher food intake per unit body weight and immature metabolic systems make them more susceptible to dietary pollutants. In this study, the team evaluated 117 mother-infant pairs (with both breast milk and maternal blood samples provided) from the LWBC to assess the health risks of PFAS intake via breast milk in 1-year-old infants and its effects on growth and development. Furthermore, 154 school-aged children (7 years old) from the cohort were followed up, fasting blood samples and dietary data from the past month were collected. Based on this, 10 most commonly consumed seafood products and 5 types of meat in the region were collected. Dietary exposure risks were evaluated by measuring PFAS concentrations in children’s serum and food samples.
The results showed that 17 traditional and emerging PFAS were detected in breast milk, with the median concentration of a traditional pollutant perfluorooctanoate (PFOA) reaching 0.970 ng/mL, significantly higher than reported values in other studies (e.g., a national survey reported a median breast milk PFAS level of 0.061 ng/mL). The median daily intake of PFOA via breast milk was 77.35 ng/kg bw, with 99.9% of exposure levels exceeding the safety limit set by the U.S. Agency for Toxic Substances and Disease Registry (ATSDR) (Figure 1).Additionally, the effects of breast milk PFAS exposure on infant growth exhibited sex-specific differences: in female infants, exposure to PFAS mixtures was significantly associated with decreased height-for-age Z-scores (HAZ) (β = -0.49; 95% CI: -0.92, -0.06) and head circumference-for-age Z-scores (HCZ) (β = -0.61; 95% CI: -1.02, -0.20), while also correlating with increased body mass index-for-age Z-scores (BMIZ) (β = 0.73; 95% CI: 0.02, 1.44). This suggests that PFAS exposure via breast milk may impair growth and increase obesity risk in female infants. No significant associations were observed in male infants (Figure 2).
Additionally, the study found widespread contamination of both traditional and emerging PFASs in local seafood. Among them, PFOA (median: 0.52 ng/g wet weight), branched perfluorooctane sulfonate (iso-PFOS) (median: 0.02 ng/g wet weight), and 6:2 chlorinated polyfluoroalkyl ether sulfonate (6:2 Cl-PFESA) (median: 0.06 ng/g wet weight) exhibited the highest concentrations among 10 linear PFAS, 8 branched isomers, and 3 emerging alternatives, respectively. Notably, the PFOA level in Zoarces slongatus (median: 87.80 ng/g wet weight), a commonly consumed fish in the region, was approximately 10 to 100 times higher than in other seafood. Children who frequently consumed this fish had significantly higher serum PFAS levels (especially PFOA) compared to those who did not (p < 0.01) (Table 1).Further analysis revealed that seafood consumption was the primary dietary exposure route for PFAS in 7-year-old children, contributing to over 80% of total exposure. The combined weekly intake of four major PFAS—PFOA, PFOS, PFNA, and PFHxS—(7.4 ng/kg bw/week) exceeded the tolerable weekly intake (TWI) recommended by the European Food Safety Authority (EFSA) (4.4 ng/kg bw/week), indicating potential health risks (Table 2).
This study extends and builds upon China’s first long-term birth cohort established in a highly polluted area, systematically evaluating dietary exposure sources of PFAS in the local population and assessing health risks for infants and school-aged children. It also identifies the main dietary contributors to PFAS exposure in children. Importantly, the findings aim to highlight the potential impact of environmental pollution on vulnerable populations (particularly breastfed infants and school-aged children) rather than to undermine the nutritional benefits of breastfeeding or seafood consumption. Based on these results, the study calls for regulatory prioritization of industrial emission controls to reduce PFAS contamination in breast milk and animal-derived foods, especially seafood, and urges the establishment of health risk standards for emerging PFAS. The findings not only provide critical scientific evidence for protecting susceptible populations in highly polluted areas and guiding intervention strategies but also serve as an important reference for shaping China’s policies on PFAS source control.
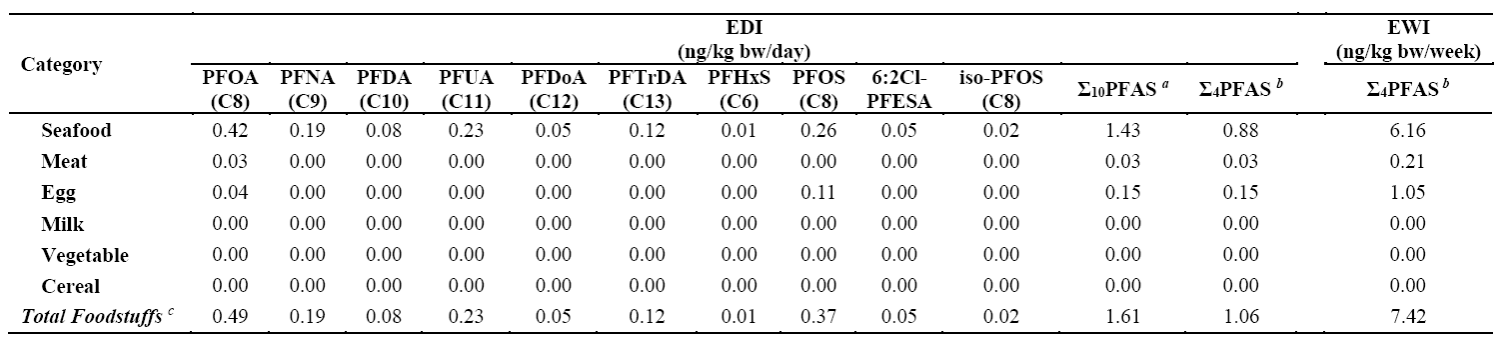
Table 1. Comparison of serum perfluoroalkyl substances (PFAS) concentrations among 7-year-old children with different dietary habits in the Laizhou Wan Birth Cohort (2010-2013) (n=124) (Unit: ng/mL)
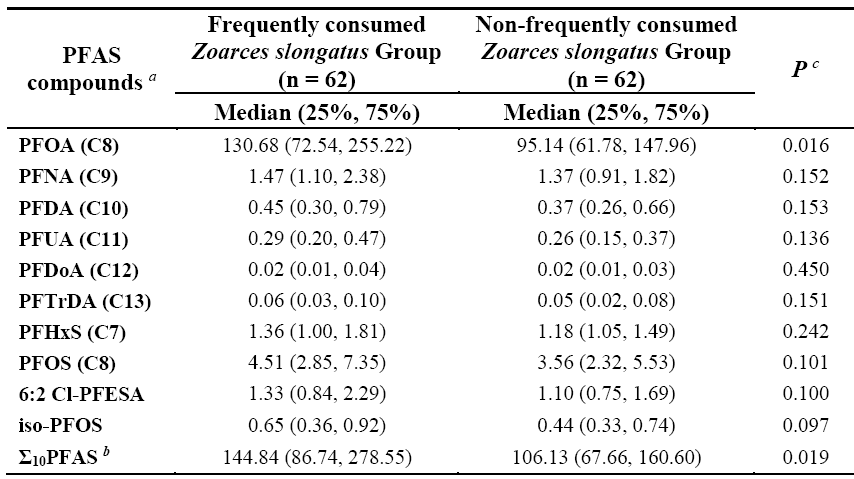
Table 2. Dietary intake assessment of perfluoroalkyl substances (PFAS) among 7-year-old children in the Laizhou Wan Birth Cohort (2010-2013)
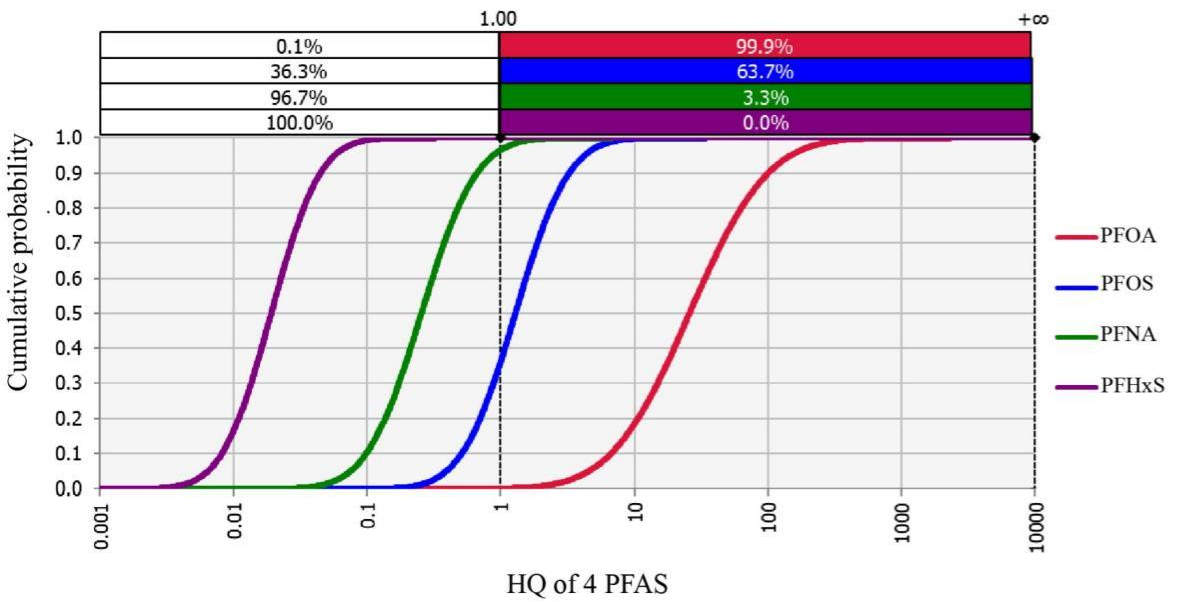
Figure 1. Cumulative probability density curves of hazard quotients (HQ) for PFOA, PFOS, perfluorononanoate (PFNA), and perfluorohexane sulfonate (PFHxS) via breast milk in the Laizhou Wan Birth Cohort (2010–2013). Note: HQ was calculated based on the minimal risk levels proposed by ATSDR in 2019.
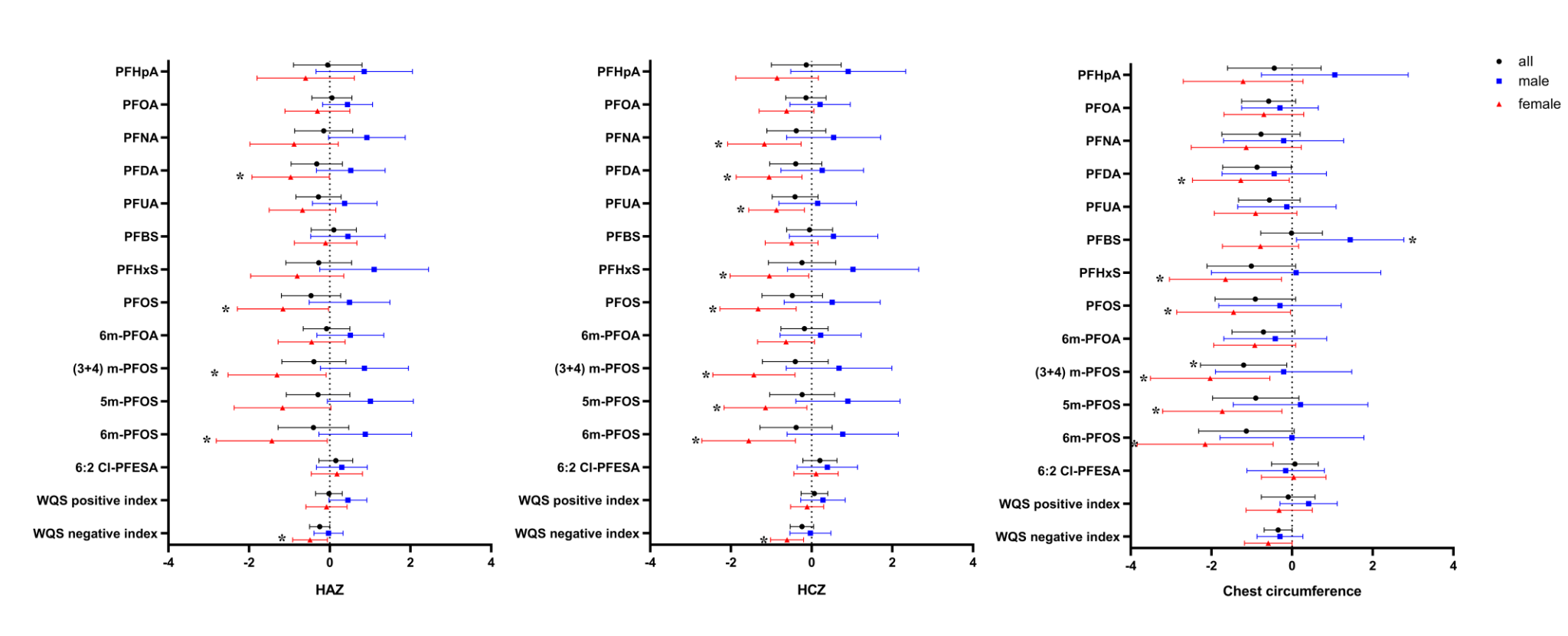
Figure 2. Association between breast milk PFAS (log10-transformed) and growth parameters (HAZ, HCZ, and chest circumference) in 1-year-old children from the Laizhou Wan Birth Cohort (2010-2013)
Note: All models were adjusted for maternal education level, parity, maternal age, and pre-pregnancy BMI. BMI, body mass index; HAZ, height-for-age Z-score; HCZ, head circumference-for-age Z-score. *: P<0.05
Author Information:
Professor Yu Gao and Professor Ying Tian from the School of Public Health at Shanghai Jiao Tong University served as co-corresponding authors for both papers. Other first/corresponding authors include Associate Researcher Yuxin Wang from the NHC Key Laboratory of Food Safety Risk Assessment, China National Center for Food Safety Risk Assessment (co-corresponding author, Environ Health Perspect, 2025), Senior Experimentalist Yan Zhang from the School of Public Health at Shanghai Jiao Tong University (first author, Environ Health Perspect, 2025), and Master's student Huyi Tao (first author, Environmental Chemistry and Ecotoxicology, 2025).
Funding:
This study was supported by the National Key Research and Development Program (2022YFC2702903, 2022YFC2705204), the National Natural Science Foundation of China (41991314, 22206032), the Science and Technology Commission of Shanghai Municipality (Grant No. 22ZR1435600), the key projects in the three-year plan of Shanghai municipal public health system (2023–2025) (GWVI-11.1-38, GWVI-11.1-41), and the Collaborative Innovation Program of Shanghai Municipal Health Commission (2020CXJQ01).




