A recent study titled “Multiplex Methionine Modulating Hydrogel for Cancer Metabolic Therapy” has been published in Advanced Materials. This work was conducted by Professor Haiyun Song’s team from the School of Public Health at Shanghai Jiao Tong University. In this research, the team develops a Multiplex Methionine Modulating Hydrogel (3M Gel) that intervenes in both extracellular methionine uptake and intracellular methionine metabolism in tumor cells. This multilayered methionine restriction strategy induces immunogenic cell death (ICD), remodels the tumor microenvironment, suppresses tumor growth, and overcomes tumor resistance to immune checkpoint blockade therapy, offering an innovative approach for cancer metabolic therapy.
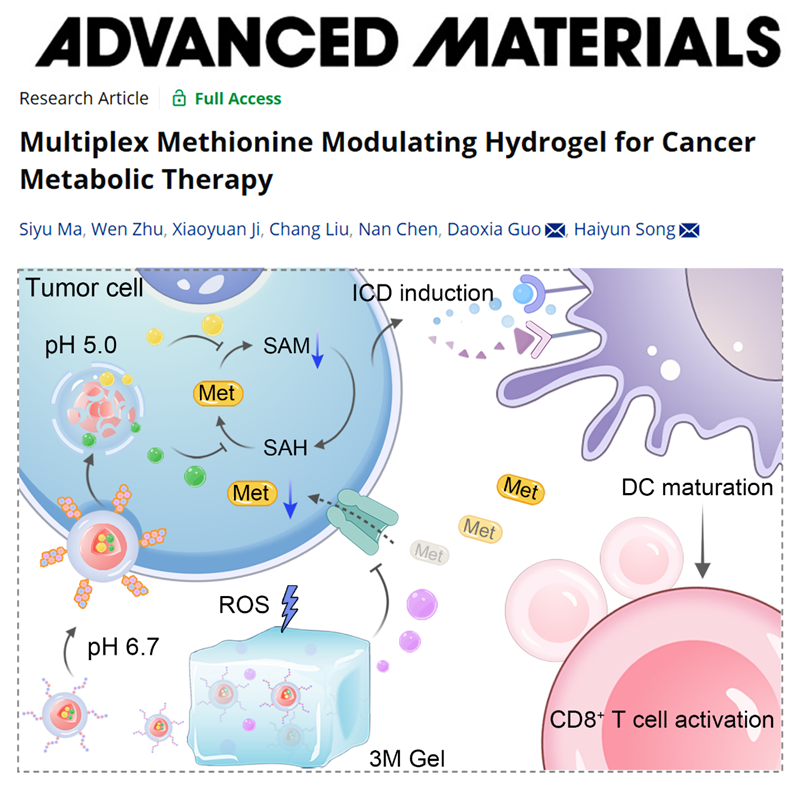
Figure 1. Schematic illustration of in situ methionine modulation by 3M Gel. Image Source: Adv. Mater.
Compared to normal cells, tumor cells exhibit remarkably higher demand for methionine and overexpress the L-type amino acid transporters (LATs) to increase exogenous methionine uptake. S-adenosylmethionine (SAM), a key product of methionine metabolism, plays a crucial role in tumorigenesis, progression, and metastasis by regulating tumor cell epigenetics, supporting polyamine synthesis, and mediating immune evasion. However, systemic methionine deprivation for SAM restriction may cause severe side effects given its extensive and essential functions in normal cells. To address this challenge, the researchers developed a localized multiplex methionine modulation strategy utilizing a tumor microenvironment (TME)-responsive hydrogel that co-delivers drug-loaded nanoparticles targeting tumor cells and free drugs, enabling precise intervention in both intracellular methionine metabolism and extracellular methionine uptake. The methionine adenosyltransferase 2A inhibitor PF9366 and adenosine dialdehyde (ADOX) were mixed with amphiphilic poly(ɛ-caprolactone)-hydrazone-poly(ethylene glycol) (PCL-Hyd-PEG) copolymers and self-assembled into acid-labile micelles, which enabled a cascaded blockade of key enzymatic reactions in the methionine cycle. These nanoparticles were camouflaged with homotypic cancer cell membranes and modified with pH-low insertion peptides (pHLIPs) for specific binding to tumor cells. The nanoparticles were then co-loaded with the LAT inhibitor JPH203 into a reactive oxygen species (ROS)-labile hydrogel, forming the 3M Gel. In the TME, the 3M Gel gradually decomposed under high ROS levels, releasing JPH203 and nanoparticles. JPH203 targeted LAT proteins on the cell membrane, restricting methionine uptake from exogenous supply, while the nanoparticles were internalized by tumor cells, disassembled in acidic organelles, and released PF9366 and ADOX for a cascaded blockade of intracellular methionine metabolism. This synergistically reduced intracellular methionine and SAM levels in tumor cells, inducing ICD and activating T cell-mediated antitumor immune responses (Figure 1).
In murine models of triple-negative breast cancer (TNBC), the locally injected 3M Gel effectively restricted SAM generation and histone methylation in tumor cells, stimulated ICD, promoted antigen-presenting cell maturation, and enhanced CD8+ T cell infiltration and activation, significantly suppressing tumor progression. By remodeling the tumor immune microenvironment, the 3M Gel overcame immune checkpoint blockade resistance in TNBC and demonstrated improved therapeutic efficacy when combined with anti-PD-1 antibodies. Furthermore, similar anti-tumor effects were observed in murine models of hepatocellular carcinoma and colorectal cancer, highlighting the broad applicability of this approach for treating various solid tumors. This study presents an innovative triple methionine modulation strategy, paving a new path for precision cancer metabolic therapy.
Professor Haiyun Song and Dr. Daoxia Guo served as the corresponding authors. Co-first authors included Dr. Siyu Ma and Wen Zhu. The study was supported by the National Key R&D Program, the National Natural Science Foundation of China, the Shanghai Natural Science Foundation, the Shanghai Municipal Health Commission, and the China Postdoctoral Science Foundation.
Original article link:
https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/adma.202420445




