On May 12, 2023, the special issue of Single-cell and ST Omics in the Journal of Pharmaceutical Analysis (IF=14.026) published online a research paper entitled “Ultrasensitive proteomics depicted an in-depth landscape for the very early stage of mouse maternal-to-zygotic transition”. This research was conducted by Hui Wang / Chen Li’s research group from the Center for Single-Cell Omics, School of Public Health, Shanghai Jiao Tong University School of Medicine, in collaboration with Zhen Liu’s research group from the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences and Innovation Institute group from the Shanghai Applied Protein Technology Co., Ltd. In this study, a Comprehensive Solution of Ultrasensitive Proteome Technology (CS-UPT) was developed for single-cell or low-input mouse oocytes and embryo samples. Using the deep coverage route, researchers provided the large-scale snapshot of the very early stage of mouse maternal-to-zygotic transition (MZT), including almost 5,500 protein groups from 20 mouse oocytes or zygotes for each sample. Moreover, significant protein regulatory networks centered on transcription factors and kinases between the MII oocyte and 1-cell embryo provided rich insights into minor zygotic genome activation (ZGA).
Fertilization and pre-implantation embryonic development in mammals is a complex biological process involving critical events, such as zygotic genome activation (ZGA) and first cell lineage differentiation. Fertilization triggers a remarkable, complex cell fate transition in oocytes from terminal differentiation to the totipotent state, termed the maternal-to-zygotic transition (MZT). Several studies based on single-cell or low-input multi-omics technologies have deciphered this complex and mysterious developmental process at the epigenetic, transcriptional, and translational levels to obtain valuable and full-scale biological insights. However, since proteins are not amplifiable, single-cell or low-input proteomic technologies have developed relatively slowly. Previous studies of mouse pre-implantation embryonic development required hundreds or thousands of mouse embryos per sample in order to obtain sufficient depth of proteomic data, raising the threshold for research in this field.
Therefore, researchers developed a Comprehensive Solution of Ultrasensitive Proteome Technology (CS-UPT) for single-cell or low-input mouse embryo samples, containing two technical routes of high-throughput or deep coverage (Fig. 1). Using the high-throughput route, researchers can obtain proteomic data from 300 single mouse zygotes as soon as 4 days, and more than 1,500 protein groups can be quantified in single mouse zygote, which provides technical support for future large-scale proteomics studies of pre-implantation embryos. Using the deep coverage route, researchers could quantify an average of 2,665 or 4,585 protein groups from single or 20 mouse zygotes, respectively; that is, using the lower embryo sample input (20 mouse zygotes) of the depth coverage technical route in CS-UPT, the number of starting embryos could be reduced 400-fold while maintaining the depth of proteome, thus substantially reducing the access threshold in this research field.
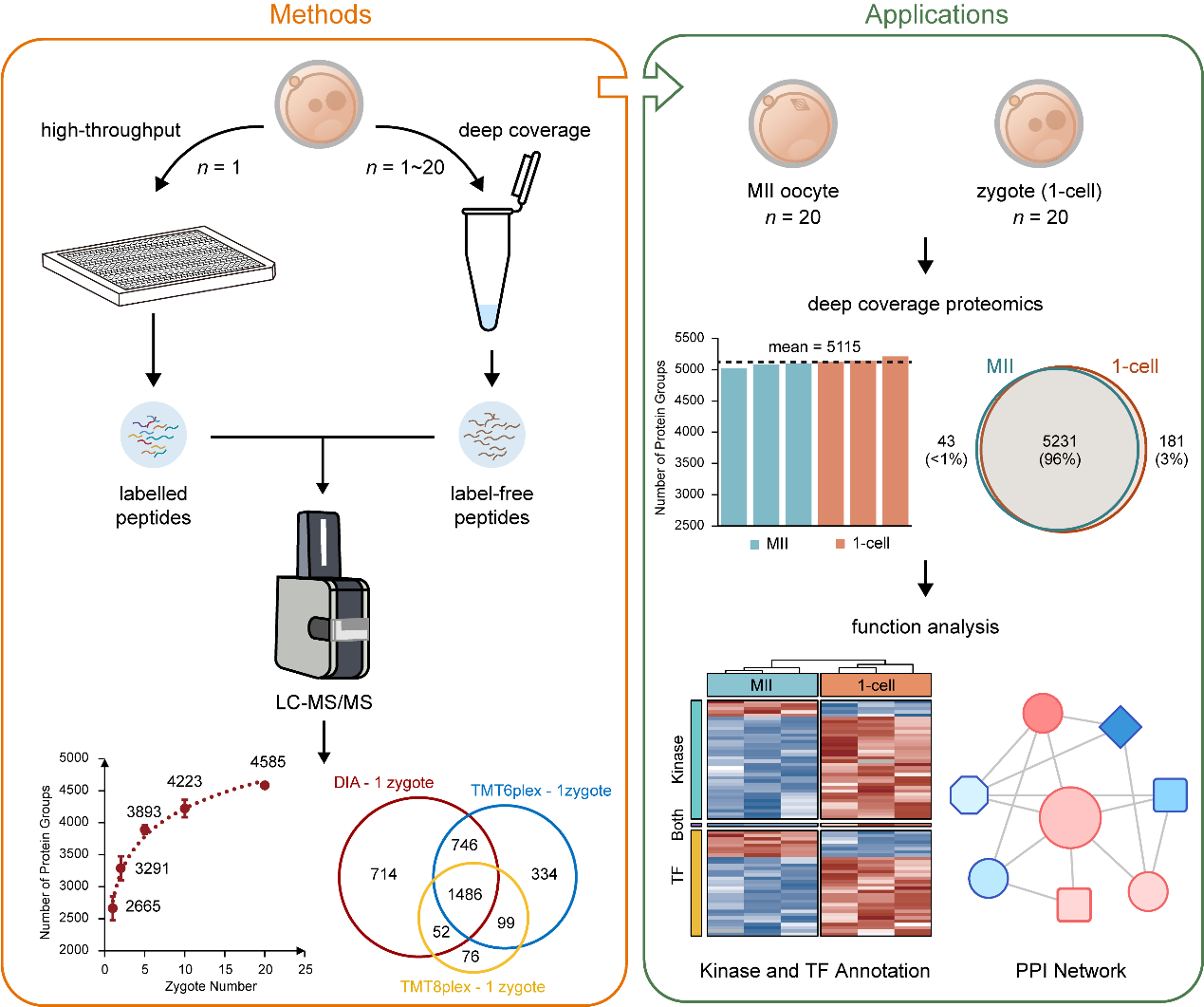
Fig. 1 The workflow and application of Comprehensive Solution of Ultrasensitive Proteome Technology (CS-UPT).
Using the deep coverage route of CS-UPT, researchers depicted an in-depth proteomic landscape of the early stage of MZT in mouse and found significant functional differences at the protein level before and after fertilization from nearly 5,500 quantitative proteomic data. Furthermore, researchers delineated a fine regulatory network around 33 transcription factors, 37 kinases, and two core components of subcortical maternal complex (SCMC) in the very early stage of MZT. Notably, most differentially expressed transcription factors, kinases, and SCMC proteins were up-regulated at the 1-cell stage, indicating that their complex protein-protein regulatory network played critical roles in minor ZGA at the 1-cell stage. In addition, in the protein-protein regulatory network, Ywhae had the most links to transcription factors and kinases or functional modules, indicating their important roles in the early stage of the MZT. In summary, these findings provide new insights for critical biological events such as MZT and minor ZGA during pre-implantation embryonic development in mouse.
Prof. Hui Wang and Prof. Chen Li from the Center for Single-Cell Omics, School of Public Health, Shanghai Jiao Tong University School of Medicine, Prof. Zhen Liu from the Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, and Dr. Ziyi Li from the Shanghai Applied Protein Technology Co., Ltd. are the corresponding authors of the paper. Research assistant Lei Gu and Ph.D. candidate Xumiao Li from the Center for Single-Cell Omics, School of Public Health, Shanghai Jiao Tong University School of Medicine, Dr. Wencheng Zhu from the Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, and Dr. Yi Shen from the Shanghai Applied Protein Technology Co., Ltd. are the co-first authors of the paper. The research was supported by grants from the Ministry of Science and Technology, National Natural Science Foundation of China (NSFC), Chinese Academy of Sciences, and Shanghai Municipal Science and Technology Commission.
Full-text link: https://www.sciencedirect.com/science/article/pii/S2095177923000916